- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Nebulized albuterol versus levalbuterol in pediatric and adult patients: A review
This article reviews the similarities and differences between albuterol (ALB) and levalbuterol (LEV) to provide P&T committees a concise summary of the available literature regarding efficacy, side effects, and cost of these agents.

Key Points
Abstract
Albuterol (ALB) is a 50:50 racemic mixture of the (R)- and (S)-isomers of albuterol, whereas levalbuterol (LEV) contains only the (R)-isomer. The (S)-isomer is considered inactive and has demonstrated increased inflammatory stimuli in vitro. Studies have compared the efficacy and side effect profiles of these 2 agents in the clinical setting. Randomized, controlled trials with adequate sample size generally have demonstrated no significant differences between LEV and ALB on the outcomes of efficacy, occurrence of adverse effects, and hospital admissions for adults and children. At this time, the use of LEV instead of ALB is not strongly supported by the literature. (Formulary. 2009;44:108–118.)
Asthma affected >25 million people in the United States during 2000.1 In the National Surveillance for Asthma in the United States conducted by the Centers for Disease Control and Prevention (CDC), the agency found that from 2001 to 2003, an average of 504,000 asthma-related hospital discharges were reported annually. During the same time frame, 1.8 million asthma-related emergency department (ED) visits occurred.2 In 2001, 12 million adults in the United States were diagnosed with chronic obstructive pulmonary disease (COPD), and an estimated 16 million had undiagnosed disease.1 These figures are only expected to increase, which will ultimately affect hospital admissions and cost.
ENANTIOMER COMPARISON
ALB is the 50:50 racemic mixture of the (R)- and (S)-isomers of albuterol, whereas LEV includes only the (R)-isomer. The (S)-isomer is considered the inactive isomer because of its limited binding to beta2-receptors, lack of bronchodilation production, and augmentation of inflammatory stimuli. The (R)-isomer is considered the active isomer because it binds to beta2-receptor sites, produces bronchodilation, and has no effect or possibly reduces inflammatory stimuli.3–8
(S)-albuterol promotes contractility through increases in intracellular calcium in airway smooth muscle cells. (S)-albuterol also enhances airway hyperresponsiveness and promotes eosinophil recruitment and activation in vitro.3,9–12 This same isomer in vitro binds to beta2-adrenergic receptors with 90 to 100 times less affinity than the (R)-isomer, and its metabolism has been demonstrated to be as much as 10 times slower than that of the (R)-isomer.4,13 These properties of the (S)-isomer may result in a decrease in the (R)-isomer's bronchodilator effects.
The (R)-isomer has been associated with adverse effects that may preclude its use in certain patients. Because beta2-receptors are also found outside the lungs, beta2-adrenergic agonists can cause tremor, headache, increased heart rate, hypokalemia, and hyperglycemia. Beta2-agonists are only available as inhaled medications, therefore reducing systemic absorption, but these side effects are concentration-dependent and so may still occur at higher doses.14

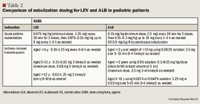
Literature Review-Adults

Lam et al17 investigated the heart rate effects of these 2 agents with a prospective, randomized, crossover study. This study included hemodynamically stable adult patients in intensive care units (ICUs) (≥48 h) who had received ≥2 consecutive doses of ALB 2.5 mg or LEV 1.25 mg every 4 hours. Patients who required a bronchodilator more often than every 4 hours, required a vasopressor or inotropic therapy, or were maintained on a beta-blocker were excluded from the study. Heart rate was recorded at the end of the second dose and at 5, 10, 15, 30, 60, 90, 120, 180, and 240 minutes after the final dose. The mean maximum increase in heart rate for patients with baseline tachycardia (n=10) was 1.4 BPM (1.3%) with ALB (not significant [NS]) and 2.0 BPM (2.1%) with LEV (NS). The mean maximum increase in heart rate for patients without baseline tachycardia (n=10) was 4.4 BPM with ALB (6.7%; P=.04) and 3.6 BPM with LEV (5%; P=.03). The authors concluded that there was no clinically significant difference between groups in maximum heart rate increases and that use of LEV was not justified in their population.17 The most important limitation of this study was its small sample size.
Another retrospective chart review performed by Truitt et al18 investigated the total number of nebulizer treatments required with ALB and LEV. The investigators assessed patient charts from a 6-month period at 1 institution. All patients with a diagnosis of asthma or COPD were included in the analysis (N=231). The only patients excluded were those with a diagnosis of cognitive disturbances or cancer. ALB 2.5 mg was administered every 4 hours and as needed, and LEV 1.25 mg was administered every 8 hours and as needed. Patients treated with LEV required fewer nebulizer treatments than patients treated with ALB (19.0±12.7 vs 30.8±24.0; P<.001), as well as fewer days of nebulizer therapy (3.9±2.3 d vs 5.5±4.3 d; P<.001). The LEV group also required fewer days of ipratropium therapy than the ALB group (2.0±2.6 d vs 4.2±4.3 d; P<.001). The mean hospital length of stay (LOS) was 5.6±4.2 days for the ALB group and 4.7±2.9 days for the LEV group (P=.058). Regression analysis controlling for diagnosis, baseline forced expiratory volume in 1 second (FEV1), and ipratropium use indicated that LEV was associated with a shorter LOS (decreased by 0.91 d; P=.015), total cost savings of $556 (P=.013), and decrease in likelihood of readmission (decreased by 67%; P=.056) compared with ALB. Because this was a retrospective review, the investigators did not consider sample size calculation and power test to be necessary.
A prospective, open-label, nonrandomized pilot study by Nowak et al19 evaluated the use of ALB and LEV in the ED. The population included 91 adult patients with asthma presenting to the ED with FEV1 of approximately 20% to 55% of predicted value. Patients received 1 of 5 doses of LEV or 1 of 2 doses of ALB every 20 minutes for 3 doses plus prednisone 60 mg (or equivalent); this was followed by a 60-minute observation period. Additional inclusion criteria were oxygen saturation ≥90%, smoking history <10 pack-years, and no other cause for wheezing or shortness of breath. Patients were excluded if they had been hospitalized for asthma within 2 months, had fixed airway disease, or had received ALB in the ED before enrollment. Patients treated with LEV 0.63, 1.25, or 3.75 mg demonstrated a median percent change in FEV1 of 13%, 56%, and 13%, respectively, after 1 dose. Patients treated with ALB 2.5 or 5 mg demonstrated a median percent change of 6% and 14%, respectively, after 1 dose. After 3 doses, FEV1 percent changes were reported to be 37%, 74%, and 26% among patients treated with LEV 0.63, 1.25, and 3.75 mg, respectively; patients treated with ALB 2.5 mg demonstrated a 39% change. A comparison of equivalent amounts of the (R)-isomer in the 2 beta2-agonists demonstrated that there were statistically significant differences. LEV 1.25 mg was associated with a significantly greater median percent change in FEV1 compared with ALB 2.5 mg after each nebulization and at 60 minutes post-treatment (P<.05). LEV 2.5 mg was also associated with a significantly greater percent change in FEV1 compared with ALB 5 mg but only at 60 minutes after treatment (P<.05). Changes in glucose, potassium, and heart rate were proportional to the dose of the (R)-isomer. Among all groups, (S)-isomer concentrations were significantly different at baseline (P<.05), and the (S)-isomer was demonstrated to be a significant confounder in the study (P=.019). Potential limitations of this study included its open-label design and the cohort sizes (12–14 patients each). Differences in age were also statistically significant among groups.
Schreck et al20 assessed the effects of LEV and ALB on hospital admission rates. Patients aged ≥1 year with a diagnosis of acute asthma requiring beta2-agonist therapy in the ED were included in the study. Patients received either LEV 1.25 mg or ALB 2.5 mg in addition to other standard treatment as needed (determined by the physician). The hospital admission rate was significantly lower in patients treated with LEV than in patients treated with ALB (4.7% vs 15.1%; P=.0016). This difference in admission rate was apparent in both the pediatric (n=234; P=.01) and adult (n=502; P=.00085) subgroups. A number of major limitations were associated with this study. First, the study was retrospective and uncontrolled. Comparisons of postdosing efficacy were not made. There were twice as many patients in the ALB group as in the LEV group, and the use of other therapy (eg, corticosteroids) was not accounted for in the study. The admission rate for patients in the ALB group was also higher than the rate observed in similar studies.
In another study by Nowak et al,21 the investigators assessed the time to ED discharge in adults presenting with an acute asthma exacerbation who received either LEV or ALB. This study was a prospective, multicenter, double-blind, parallel-group trial. Patients were included if they were aged ≥18 years and presented to the ED or clinic with acute exacerbation of asthma and with FEV1 of 20% to 55% of predicted, ≥6-month history of physician-diagnosed asthma, ≥90% oxygen saturation, ≤6 L of supplemental oxygen, and no other known cause of wheezing or shortness of breath. Patients excluded from the trial were those with sufficient disease severity to avoid delayed treatment, those who received therapy other than oxygen after ED/clinic arrival, those with a history of severe asthma within the previous 12 months, those who had been treated for acute asthma within 2 weeks or hospitalized within 1 month of enrollment, and those with a ≥10-pack-year smoking history. Patients received either LEV 1.25 mg or ALB 2.5 mg every 20 minutes for 3 doses and then every 40 minutes for ≤3 more doses. Patients could receive additional asthma therapy if this was considered necessary by the investigator. All patients were treated with prednisone 40 mg, and during the initial 3-hour treatment period, certain medications were not permitted, including ipratropium, long-acting beta2-agonists (LABAs), leukotriene modifiers, methylxanthines, corticosteroids other than prednisone 40 mg, and magnesium. At the time of discharge from the ED, study participants were given prescriptions for 5 days of corticosteroids, scheduled and as-needed beta2-agonist therapy for 3 days, and as-needed beta2-agonist therapy ≤3 times/d for 7 days. There were 2 evaluation periods in this study: an acute period and a 7- and 30-day follow-up period. The median time to discharge (primary outcome) was similar for both groups (LEV, 76.0 min vs ALB, 78.5 min; P=.74). The investigators observed no statistical differences between groups for overall admission rate (P=.28) or for relapse rate at 7 days and 30 days. The increase in FEV1 after the first dose was significantly greater in LEV-treated patients than in ALB-treated patients (0.5±0.43 L vs 0.43±0.37 L; P=.02), but the difference was not significant when comparing ALB and LEV for doses 1 to 3 (P=.093) and doses 1 to 6 (P=.113). Subgroup analysis demonstrated a benefit of LEV compared with ALB on FEV1 and hospital admission rate in patients who were not taking corticosteroids at home, but no difference was observed among patients taking corticosteroids. In patients with the highest (S)-isomer concentrations, LEV was associated with significant improvements in FEV1 and fewer admissions when compared with ALB. No difference was observed between groups for the incidence of adverse effects, including tachycardia. The authors concluded that there is a benefit in using systemic corticosteroids early in therapy and scheduled dosing of beta2-agonists in patients with acute exacerbations. The major limitation in this study was physicians' use of subjective criteria (80%) more often than objective criteria (20%) in deciding which patients to discharge.
Overall, there are many limitations in the ALB/LEV studies in adult patients. Three of the studies are retrospective in nature, limiting their usefulness in clinical practice and in literature reviews for P&T committees.16,18,20 Two of the prospective trials had a small sample size.17,19 Only 1 of the studies was large, prospective, and double blind.21 The studies do not demonstrate that LEV consistently improves FEV1 to a greater extent than ALB.19,21 Even when an increase in heart rate was statistically higher in ALB-treated patients than in LEV-treated patients, the maximum increase was only 2 to 3 BPM with ALB.16,17,21 One of the prospective trials demonstrated that there were no statistically significant differences between groups in side effects.21 High levels of the (S)-isomer may have a negative effect on the usefulness of either agent, particularly ALB.19,21 Studies need to be conducted to further research the effects of high (S)-isomer concentrations and the use of corticosteroids before arrival at the ED or hospital.
LITERATURE REVIEW-PEDIATRICS

A multicenter, randomized, double-blind study published by Milgrom et al23 compared the efficacy and safety of LEV and ALB in 338 stable asthmatic children. Patients were included if they had a diagnosis of at least mild asthma ≥60 days before screening, were aged 4 to 11 years, and had baseline FEV1 within 40% to 85% of predicted with ≥15% reversibility with ALB at screening. Patients who had a lower respiratory tract infection within 2 weeks of randomization or had a clinically significant abnormality in electrocardiogram were not included in the study. Patients were allowed to continue stable doses of inhaled corticosteroids that were initiated ≥60 days before randomization. Patients were treated with LEV 0.31 or 0.63 mg, ALB 1.25 or 2.5 mg, or placebo 3 times/d for 21 days. The investigators observed no significant difference in the primary outcome of peak percent change from baseline in FEV1 at 21 days between LEV 0.31 and 0.63 mg and ALB 2.5 mg (LEV 0.31 mg, 24.9%; LEV 0.63 mg, 25.4%; ALB 2.5 mg, 26.7%; P=NS for LEV vs ALB; P<.05 for LEV vs placebo and ALB vs placebo). No differences were observed among treatment groups on the outcomes of asthma symptom assessment score, symptom-free days, quality-of-life score, rescue medication use, and time to peak improvement in FEV1. Serum glucose was significantly increased with ALB 2.5 mg compared with LEV 0.31 and 0.63 mg on Days 0 and 21 (P≤.043). The authors concluded that smaller doses of LEV led to bronchodilation similar to that of standard-dose ALB in this group of patients. They recommended that for patients aged 4 to 11 years, the starting dose of LEV should be 0.31 mg.
A third study evaluating the comparative efficacy of LEV and ALB in stable asthmatic children was carried out by Skoner et al.24 This study was a randomized, multicenter, double-blind trial that evaluated the treatment response of 176 children with a history of asthma and no other chronic disease states. Patients eligible for enrollment were aged 2 to 5 years, diagnosed with asthma for ≥30 days, in good health, had no chronic medical conditions, and had a thoracic radiograph with results suggesting no other pulmonary disorder. Treatment with a systemic corticosteroid within 30 days or astemizole within 90 days, a respiratory tract infection within 2 weeks, or life-threatening asthma within 1 year of the study screening were exclusion criteria. Patients were allowed to continue treatment with ipratropium and inhaled corticosteroids if these agents were taken at stable doses before and throughout the study. LEV 0.31 or 0.63 mg, ALB 1.25 (weight <33 lbs) or 2.5 mg (weight ≥33 lbs), or placebo were administered 3 times daily for 3 weeks, and patients were then evaluated with a new questionnaire (Pediatric Asthma Questionnaire [PAQ]). The investigators observed no differences among groups on the primary outcome of change in PAQ total score (P=NS). The Pediatric Asthma Caregiver's Quality of Life Questionnaire (PACQLQ) was also administered, and significant improvements were observed in patients <33 pounds who received LEV (P<.05). Significant improvements in this outcome were not noted in other groups versus placebo. No significant differences were observed between LEV and ALB for rescue medication use and adverse effects, and investigators noted no differences in peak expired flow rate (PEF) for LEV and ALB versus placebo at Weeks 2 and 3. Only at Day 0 did all ALB and LEV groups demonstrate statistically significant differences in PEF compared with placebo. On Day 7, only the LEV groups demonstrated improvement in PEF over placebo (P<.05). Of note, the PAQ was an unvalidated questionnaire, and no statistical calculation was performed to determine appropriate sample size. Furthermore, patients were evaluated only during a short, 3-week study period, and the subgroups were small because of the weight-based dosing of the ALB group. Additionally, PEF is a difficult measurement to use in the pediatric population, as some patients are unable to provide accurate measurements.
Carl et al25 compared hospital admission rates from the ED in a randomized, double-blind, controlled trial in 482 children who presented to the ED with an acute asthma exacerbation. Patients were included if they were aged 1 to 18 years and had previously been diagnosed with asthma. Patients not currently being treated for asthma and those treated at other institutions before ED presentation were excluded. Study patients received either LEV 1.25 mg or ALB 2.5 mg every 20 minutes until they met discharge criteria or until they reached the maximum of 6 doses in 2 hours, in which case they were admitted to the hospital. Patients also received oral prednisone if they did not meet discharge criteria after the first nebulized beta2-agonist in the ED (2 mg/kg/d; maximum, 60 mg). Oxygen was administered to all patients who required it to maintain an oxyhemoglobin saturation of 94%. Any patients who required treatment for severe respiratory distress received the study drug in addition to ipratropium and epinephrine as part of the standardized intensification regimen. For the primary outcome measure of hospital admission rate, patients in the ALB group demonstrated a higher admission rate than those in the LEV group (45.4% vs 36.3%; P=.02). Patients who had ≥2 ALB doses in the 2 hours before presentation to the ED had a 40% higher hospital admission rate compared with patients who had not received ≥2 doses (RR=1.39; 95% CI, 1.13–1.72; P=.02). No significant difference in secondary outcomes, including ED LOS (P=.25), hospital LOS (P=.63), and number of treatments required in the ED (P=.08) was observed between groups. No significant adverse effects were observed in either group, and no significant difference between groups was observed for mean heart rate (P=.94), respiratory rate (P=.26), or oxyhemoglobin saturation (P=.81). This study has not been validated in any other prospective trials to date. This trial also had unusually high admission rates for asthma patients, and no objective criteria for hospital admission were specified. The LEV group also contained more patients taking cromolyn at baseline than the ALB group (P=.002).
Qureshi et al26 carried out a prospective, double-blind, randomized, controlled study in 129 children aged 2 to 14 years with acute asthma exacerbation presenting to the ED. Additional inclusion criteria were history of asthma and a clinical asthma score of >8 or FEV1 <70% of predicted. Exclusion criteria included specific medication use, history of chronic underlying lung disease, and history of heart disease. Study participants were administered weight-based doses of either LEV or ALB at 20-minute intervals for 3 doses, followed by doses at 30- to 60-minute intervals as needed. All patients received prednisone 2 mg/kg or equivalent with the second treatment, and ipratropium therapy could begin after the third treatment. Study participation was complete at disposition or after 5 treatments. No difference was observed between weight-based doses of LEV and ALB after the first, third, and fifth nebulizer treatments on the primary outcome of improvement in clinical asthma score or percentage of predicted FEV1. Investigators also observed no difference between groups on the secondary outcomes of median number of nebulizer treatments administered (3 for both groups); length of care (LEV, 121 min; ALB, 125 min); and changes in pulse rate, respiratory rate, and oxyhemoglobin saturation. This study was not powered to detect a difference in hospitalization rates of patients; however, the observed rate of hospitalization was 11% in the LEV group and 13% in the ALB group compared with 36% to 46% in the Carl et al25 study. Because of their age, many participants were unable to reliably perform pulmonary function tests, which could have affected the results.
Hardasmalani et al27 conducted a randomized, double-blind, prospective trial that included 70 children aged 5 to 21 years with a known history of asthma who presented to the ED with an acute asthma exacerbation. Patients were excluded from the study if they had chronic cardiac, neurologic, or endocrinologic disorders; were allergic to beta-agonists; or had received ≥2 treatments in the previous 2 hours. Study patients received either LEV 1.25 mg or ALB 2.5 mg plus ipratropium (<30 kg, 0.25 mg; >30 kg, 0.5 mg) for 3 doses at 20-minute intervals. No differences between LEV and ALB were observed for physiologic parameters, including oxygen saturation change (LEV, 1.42%±1.54%; ALB, 1.41%±1.76%; P=.990), respiratory rate change (LEV, –3.77±6.02 breaths/min; ALB, –3.50±3.84 breaths/min; P=.830), peak flow rate change (LEV, 95.14±63.06 L; ALB, 97.35±77.55 L; P=.707), or peak flow rate percent change (LEV, 66.03%±41.67%; ALB, 70.37%±53.89%; P=.707). Additionally, there was no difference between groups for hospital admission rate from the ED (LEV, 8.3%; ALB, 5.9%; P=.158). This study had a small number of patients, but the rate of hospital admissions was again much lower than the rate observed in the Carl et al25 trial.
As described earlier, Schreck et al20 also evaluated hospital admission rates in their retrospective chart review of patients aged ≥1 year and observed a lower hospital admission rate in patients treated with LEV (LEV, 4.7%; ALB, 15.1%; P=.0016). This study, however, had many limitations (see earlier discussion).
Ralston et al28 evaluated 140 patients aged 6 to 18 years presenting to the ED in a prospective, randomized, double-blind, controlled trial. Patients were included in the study if they had a history of asthma, were able to use a peak flow meter, and had PEF <80% of predicted on presentation to the ED. Excluded patients were those with impending or actual respiratory distress and those who had undergone treatment with LEV or ipratropium within 6 hours of study enrollment. Patients received either LEV 1.25 mg for ≤6 doses or the combination of ALB 5 mg and ipratropium 0.25 mg for ≤3 doses and then ALB alone for ≤3 more doses. A follow-up telephone interview within 72 hours of discharge from the ED was performed to ascertain the occurrence of unplanned medical visits. For the primary end point of ED LOS, there was no significant difference between groups (LEV, 80 min; ALB, 94 min; P=.130). The authors reported no difference between groups in percent change in PEF (P=.365), number of nebulizer treatments required (P=.718), new symptoms of asthma (P=.326), or frequency of unplanned return visits within 72 hours (P=1.00). Patients treated with ALB plus ipratropium required fewer adjunctive medications than patients treated with LEV (ALB, 13%; LEV, 29%; P=.022). Patients treated with ALB plus ipratropium did demonstrate a significant mean increase in heart rate compared with LEV-treated patients (ALB, 26 BPM; LEV, 10 BPM; P<.001), but the dose of albuterol used was 5 mg. This is twice the dose normally used, whereas LEV 1.25 mg is half the comparative dose. The use of PEF as an outcome measure could be a limitation of the study, as PEF is not as sensitive as FEV1 in assessing lung function.
Of the prospective clinical trials, 3 evaluated LEV and ALB in stable asthma patients.22–24 No change was demonstrated in FEV1 from baseline for either beta2-agonist.22,23 These 3 studies also demonstrated no statistically significant differences between LEV and ALB in the outcomes of desensitization on Day 21, time to peak improvement in FEV1, overall asthma symptoms assessment score, symptom-free days, quality-of-life score, and rescue medication use.23 Differences in heart rate appeared to be nonsignificant except when comparing larger doses of the (R)-isomer with smaller doses.22–24 Four prospective studies reviewed the use of LEV and ALB in the acute care setting.25–28 One of the trials demonstrated no difference between the 2 medications for the outcome of change in FEV1, and another trial did not demonstrate a difference in peak flow rate.26,27 Of all the parameters measured in these 4 trials, LEV demonstrated benefit in 2 areas only: hospital admission rate from ED and change in heart rate.25,28 Carl et al25 demonstrated a benefit with LEV on admission rate, but the study did not use objective admission criteria, and its high admission rate has not been reproduced in any other trial. Heart rate was demonstrated to be higher in patients taking ALB 5 mg than in those taking LEV 1.25 mg.28 Again, larger doses of the (R)-isomer were linked with greater increases in heart rate.28 Differences in heart rate and all other measured side effects, including respiratory rate, were not statistically significant in any other trial.25–27
ASTHMA AND COPD GUIDELINES
The 2007 asthma guidelines state that LEV offers no advantage over ALB in terms of improved efficacy or safety in the treatment of acute exacerbations (evidence-based: B).29 The 2008 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines also state that for single-dose beta2-agonists as needed in COPD, there appears to be no advantage in using LEV over conventional nebulized bronchodilators.30
ADVERSE EFFECTS
Adverse effects are similar for both beta2-agonists. A review of the official prescribing information suggests that no significant differences in important adverse effects were observed when equipotent doses of ALB and LEV were compared in controlled clinical trials. Adverse effects studied included tachycardia, migraine, dizziness, nervousness, tremor, anxiety, elevations in blood glucose, and decreases in potassium.15,31
PRICING
Average wholesale pricing (AWP) for ALB is approximately $1.21 per 2.5-mg dose. The comparable dose of LEV (1.25 mg) is $4.26 per unit dose.32 The national average cost of a respiratory therapist per nebulizer treatment is approximately $6.18 per 15-minute treatment, based on averages from the Bureau of Labor Statistics.33 When the acquisition cost for medication and respiratory therapist salary is added together, the average cost of a nebulizer treatment is approximately $7.39 for ALB and $10.44 for LEV (excludes the nebulizer and nebulizer compressor).32,33 Although these costs may be skewed, as most institutions receive more competitive pricing through their wholesaler, they do underscore the fact that LEV costs are at least triple the costs of ALB. Asmus and Hendeles34 estimated that a single dose of LEV 1.25 mg costs approximately 5 times more than ALB 2.5 mg.34 Truitt et al18 demonstrated that LEV reduces overall costs compared with ALB when LEV is dosed correctly (3 times/d) and requires no or fewer as-needed doses.18 However, personal experience in a community hospital has demonstrated that it is common for physicians to dose LEV in the same way that ALB would be dosed (ie, every 2–4 h). For patients with acute exacerbations, even more frequent dosing of LEV may be used until the patient is stabilized.15 More aggressive treatments with beta2-agonists are needed because the dose-response curve is shifted to the right during acute exacerbations of asthma, requiring larger doses of the medications.34 These practices result in higher medication costs for LEV when compared with ALB.
Reimbursement for ALB and LEV by the Centers for Medicare and Medicaid Services (CMS) has evolved over time. The most recent change occurred in April 2008, when albuterol and levalbuterol were reestablished with separate Healthcare Common Procedure Coding System codes. Medicare determined that it would reimburse ALB at $0.044 per milligram and LEV at $0.280 per 0.5 milligrams. Based on widely available market prices, use of ALB alone would probably result in breaking even, whereas use of LEV would result in a loss (ALB 2.5 mg, $0.11–$0.1025=$0.0075; LEV 1.25 mg, $0.70–$3.045=$–2.345).35
CONCLUSION
Despite data suggesting that the (R)-isomer of ALB is the active isomer and the presence of the (S)-isomer may only induce adverse effects, published randomized, controlled trials with adequate sample sizes generally have demonstrated no significant differences between LEV and ALB in efficacy, occurrence of adverse effects, or hospital admissions. Studies that demonstrated benefits with LEV were often retrospective in design, which require validation with a randomized, controlled trial. One study also demonstrated a decreased ED admission rate in pediatric patients with the use of LEV, but this finding has yet to be validated.
LEV is more potent than ALB, but similar efficacy is observed at equipotent doses. There is also no evidence of a greater increase in heart rate with ALB compared with LEV. The use of LEV in the adult or pediatric population is not strongly supported by the available literature. In addition, LEV does not demonstrate benefit over ALB based on comparison of drug cost versus CMS reimbursement, especially considering normal prescribing practices.
Dr Crader is an assistant professor of pharmacy practice, University of Arkansas for Medical Sciences College of Pharmacy, Little Rock. Dr Borkowski is the pharmacy clinical coordinator, OSF St. Anthony Medical Center, Rockford, Illinois.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
REFERENCES
1. Kruzikas DT, Jiang HJ, Remus D, Barrett ML, Coffey RM, Andrews R. Respiratory diseases: Adult asthma, pediatric asthma, and chronic obstructive pulmonary disease. In: Preventable hospitalizations: Window into preventative and primary care, 2000. US Department of Health and Human Services. AHRQ HCUP Fact Book No. 5: 30–33.
2. Moorman JE, Rudd RA, Johnson CA, et al. National surveillance for asthma-United States, 1980–2004. MMWR Surveill Summ. 2007;56:1–54.
3. Mitra S, Ugur M, Ugur O, Goodman HM, McCullough JR, Yamaguchi H. (S)-albuterol increases intracellular free calcium by muscarinic receptor activation and a phospholipase C-dependent mechanism in airway smooth muscle. Mol Pharmacol. 1998;53:347–354.
4. Penn RB, Frielle T, McCullough JR, Aberg C, Benovic JL. Comparison of R-, S-, and RS-albuterol interaction with human beta 1- and beta 2-adrenergic receptors. Clin Rev Allergy Immunol. 1996;14:37–45.
5. Lötvall J, Palmqvist M, Arvidsson P, Maloney A, Ventresea GP, Ward J. The therapeutic ratio of R-albuterol is comparable with that of RS-albuterol in asthmatic patients. J Allergy Clin Immunol. 2001;108:726–731.
6. Ameredes BT, Calhoun WJ. Modulation of GM-CSF release by enantiomers of beta-agonists in human airway smooth muscle. J Allergy Clin Immunol. 2005;116:65–72.
7. Agrawal DK, Ariyarathna K, Kelbe PW. (S)-albuterol activates pro-constrictory and pro-inflammatory pathways in human bronchial smooth muscle cells. J Allergy Clin Immunol. 2004;113:503–510.
8. Volcheck GW, Kelkar P, Bartemes KR, Gleich GJ, Kita H. Effects of (R)- and (S)-isomers of beta-adrenergic agonists on eosinophil response to interleukin-5. Clin Exp Allergy. 2005;35:1341–1346.
9. Yamaguchi H, McCullough JR. S-albuterol exacerbates calcium responses to carbachol in airway smooth muscle cells. Clin Rev Allergy Immunol. 1996;14:47–55.
10. Johansson F, Rydberg I, Aberg G, Andersson RG. Effects of albuterol enantiomers on in vitro bronchial reactivity. Clin Rev Allergy Immunol. 1996;14:57–64.
11. Frieri M, Pergolizzi R, Millan C, Dominguez PF. Cytokine, chemokine, and nitric oxide (NO) release in stimulated small airway epithelial cells (SAEC) treated with beta2-agonist enantiomers of albuterol [abstract]. J Allergy Clin Immunol. 2000;105(suppl):S292. Abstract 858.
12. Volcheck GW, Gleich GJ, Kita H. Pro-and anti-inflammatory effects of beta adrenergic agonists on eosinophil response to IL-5. J Allergy Clin Immunol. 1998;101:S35.
13. Walle T, Eaton EA, Walle UK, Pesola GR. Stereoselective metabolism of RS-albuterol in humans. Clin Rev Allergy Immunol. 1996;14:101–113.
14. Bennett JA, Tattersfield AE. Time course and relative dose potency of systemic effects from salmeterol and salbutamol in healthy subjects. Thorax. 1997;52:458–464.
15. Thompson Healthcare. Micromedex Healthcare Series (2008). http://www.thomsonhc.com/. Accessed March 24, 2009.
16. Scott VL, Frazee LA. Retrospective comparison of nebulized levalbuterol and albuterol for adverse events in patients with acute airflow obstruction. Am J Ther. 2003;10:341–347.
17. Lam S, Chen J. Changes in heart rate associated with nebulized racemic albuterol and levalbuterol in intensive care patients. Am J Health Syst Pharm. 2003;60:1971–1975.
18. Truitt T, Witko J, Halpern M. Levalbuterol compared to racemic albuterol: Efficacy and outcomes in patients hospitalized with COPD and asthma. Chest. 2003;123:128–135.
19. Nowak RM, Emerman CL, Schaefer K, Disantostefano RL, Vaickus L, Roach JM. Levalbuterol compared with racemic albuterol in the treatment of acute asthma: Results of a pilot study. Am J Emerg Med. 2004;22:29–36.
20. Schreck DM, Babin S. Comparison of racemic albuterol and levalbuterol in the treatment of acute asthma in the ED. Am J Emerg Med. 2005;23:842–847.
21. Nowak R, Emerman C, Hanrahan JP, et al; XOPENEX Acute Severe Asthma Study Group. A comparison of levalbuterol with racemic albuterol in the treatment of acute severe asthma exacerbations in adults. Am J Emerg Med. 2006;24:259–267.
22. Gawchik SM, Saccar CL, Noonan M, Reasner DS, DeGraw SS. The safety and efficacy of nebulized levalbuterol compared with racemic albuterol and placebo in the treatment of asthma in pediatric patients. J Allergy Clin Immunol. 1999;103: 615–621.
23. Milgrom H, Skoner DP, Bensch G, Kim KT, Claus R, Baumgartner RA; Levalbuterol Pediatric Study Group. Low-dose levalbuterol in children with asthma: Safety and efficacy in comparison with placebo and racemic albuterol. J Allergy Clin Immunol. 2001;108:938–945.
24. Skoner DP, Greos LS, Kim KT, Roach JM, Parsey M, Baumgartner RA. Evaluation of the safety and efficacy of levalbuterol in 2-5-year-old patients with asthma. Pediatr Pulmonol. 2005;40:477–486.
25. Carl JC, Myers TR, Kirchner HL, Keresmar CM. Comparison of racemic albuterol and levalbuterol for treatment of acute asthma. J Pediatr. 2003;143:731–736.
26. Qureshi F, Zaritsky A, Welch C, Meadows T, Burke BL. Clinical efficacy of racemic albuterol versus levalbuterol for the treatment of acute pediatric asthma. Ann Emerg Med. 2005;46:29–36.
27. Hardasmalani MD, DeBari V, Bithoney WG, Gold N. Levalbuterol versus racemic albuterol in the treatment of acute exacerbation of asthma in children. Pediatr Emerg Care. 2005;21:415–419.
28. Ralston ME, Euwema MS, Knecht KR, Ziolkowski TJ, Coakley TA, Cline SM. Comparison of levalbuterol and racemic albuterol combined with ipratropium bromide in acute pediatric asthma: A randomized controlled trial. J Emerg Med. 2005;29:29–35.
29. National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007 [erratum in J Allergy Clin Immunol. 2008;121:1330]. J Allergy Clin Immunol. 2007;120(5 suppl):S94–138.
30. Global Initiative for Chronic Obstructive Pulmonary Disease: Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2008). http://www.goldcopd.com/. Accessed March 30, 2009.
31. Xopenex [package insert]. Marlborough, MA: Sepracor Inc.; 2005.
32. Red Book. February 2009 update. Montvale, NJ: Thomson PDR; 2009.
33. Bureau of Labor Statistics. Occupational outlook handbook, 2008–09 edition: Respiratory therapists. United States Department of Labor. http://www.bls.gov./oco/ocos084.htm. Accessed March 24, 2009.
34. Asmus MJ, Hendeles, L. Levalbuterol nebulizer solution: Is it worth five times the cost of albuterol? Pharmacotherapy. 2000;20:123–129.
35. Department of Health and Human Services Office of Inspector General. Comparison of average sales prices to widely available market prices for inhalation drugs. July 2008. http://www.oig.hhs.gov/oei/reports/oei-03-07-00190.pdf. Accessed March 24, 2009.
