- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Rheumatoid arthritis drug poses fatal side effects
FDA is warning about an increased risk of death and blood clots in the lungs in patients with rheumatoid arthritis taking a specific dosage of this drug.
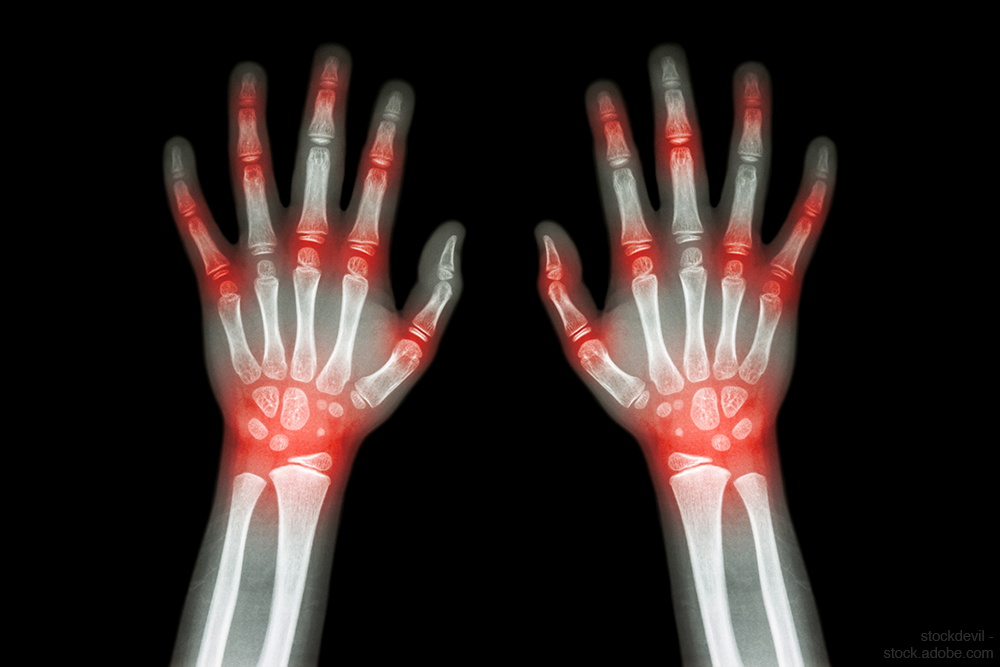
FDA is warning about an increased risk of death and blood clots in the lungs in patients with rheumatoid arthritis taking a specific dosage of tofacitinib (Xeljanz, Xeljanz XR, Pfizer).
In a new safety alert, FDA said that a clinical safety trial found that, when a 10 mg twice-daily dose of Xeljanz was used in patients with RA, there is an increased risk of death and blood clots. FDA has not approved this 10 mg twice-daily dose for RA; it is only approved in the dosing regimen for patients with ulcerative colitis.
Related: FDA: Higher death risk with this gout drug
When FDA first approved Xeljanz, it required a clinical trial of RA patients to evaluate the risk of heart-related events, cancer, and opportunistic infections with the medicine at two doses (10 mg twice daily and 5 mg twice daily) in combination with methotrexate, compared to a tumor necrosis factor (TNF) inhibitor.
“During the most recent analysis of the trial, an external data safety monitoring committee found an increased occurrence of blood clots in the lungs and death in patients treated with tofacitinib 10 mg twice daily compared to patients treated with tofacitinib 5 mg twice daily or a TNF inhibitor,” FDA said in the alert.
Related: Startling number of patients inappropriately prescribed fentanyl
Healthcare professionals should monitor patients for the signs and symptoms of pulmonary embolism, and advise them to seek medical attention immediately if they experience them, FDA said.
Patients should not stop or change their dose of Xeljanz without first talking to their healthcare professional.
Patients should seek medical attention immediately if they experience symptoms of a blood clot in their lungs or other unusual symptoms such as sudden shortness of breath or difficulty breathing, chest pain or back pain, coughing up blood, excessive sweating, and clammy or bluish colored skin.
Read more: FDA warns maker of ingredient in huge heart drug recall
Drugs to Watch: Mental Health Conditions
April 11th 2024The FDA is reviewing two novel therapies: a psychedelic-assisted therapy for PTSD with a target action date of Aug. 11, 2024, and therapy for schizophrenia that does not directly block dopamine receptors with an action date of Sept. 26, 2024.
