- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
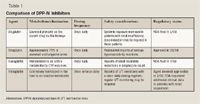
Alogliptin: A dipeptidyl peptidase-IV inhibitor for the treatment of type 2 diabetes
Alogliptin is a highly selective DPP-IV inhibitor under investigation for the treatment of type 2 diabetes. An NDA for alogliptin was submitted in January 2008, and a response from FDA is expected in the fourth quarter of this year.

Key Points
Abstract
Dipeptidyl peptidase-IV (DPP-IV) inhibitors are a promising new class of drugs that may potentially play an important role in the management of type 2 diabetes mellitus. These agents improve glycemic control by preventing the rapid inactivation of the incretin hormones glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP), leading to stimulation of insulin release from the pancreas, inhibition of glucagon secretion, and potentially an improvement in pancreatic beta-cell function. Alogliptin is a highly selective DPP-IV inhibitor under investigation for the treatment of type 2 diabetes. Alogliptin's efficacy as monotherapy or in combination with other antidiabetic agents has been demonstrated in several randomized, placebo-controlled trials. Treatment with alogliptin for 26 weeks resulted in a significant reduction in hemoglobin A1c (HbA1c) in patients with diabetes inadequately controlled with their current regimen. In these trials, alogliptin was well tolerated, with a negligible incidence of hypoglycemia and no significant changes in weight. However, the safety of long-term therapy is currently unknown. An NDA for alogliptin was submitted in January 2008, and a response from FDA is expected in the fourth quarter of this year. If approved, alogliptin will compete with sitagliptin, the only DPP-IV inhibitor currently approved by FDA, and with other agents under review by FDA (vildagliptin and saxagliptin). Long-term safety and efficacy trials and further clinical experience will determine the clinical use of this drug and its potential place in therapy. (Formulary. 2008;43:317–325.)
Diabetes mellitus is a chronic, progressive disease affecting >180 million people worldwide; it is estimated that this disease will affect >360 million people by 2030.1 The majority of these patients have type 2 diabetes (formerly called non-insulin-dependent diabetes). In the United States, >23 million patients have diabetes. The costs associated with diabetes and its complications are staggering. According to Centers for Disease Control and Prevention (CDC) estimates, total costs (direct and indirect) attributable to diabetes in 2007 were $174 billion.2 The incidence of diabetes and its associated costs are likely to increase in light of the obesity epidemic.
Despite the availability of several therapeutic options, the majority of patients with diabetes fail to achieve their target HbA1c goal.9 Currently, several new pharmacologic approaches to treatment are being explored. An ideal drug would be effective in lowering HbA1c, would have no drug-drug interactions with other antidiabetic medications, would not cause weight gain, and would have low risk for hypoglycemia.
DPP-IV inhibitors have been demonstrated to improve glycemic control in patients with type 2 diabetes; these agents have also been demonstrated to improve beta-cell function in animal studies and may have similar effects in humans.14,15 Further studies are needed to examine their potential use in preventing type 2 diabetes in patients with impaired glucose tolerance and in modifying the progression of type 2 diabetes.
Alogliptin is a selective DPP-IV inhibitor under investigation for the treatment of type 2 diabetes. An NDA for alogliptin was submitted in January 2008. A response from FDA is expected in the fourth quarter of 2008.
CHEMISTRY AND PHARMACOLOGY
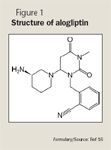
Inhibition of DPP-IV is a newer therapeutic approach for the treatment of type 2 diabetes in which the endogenous incretin hormones are targeted. These hormones are released from endocrine cells in the gastrointestinal (GI) tract when they are stimulated by intraluminal glucose. The hormones regulate glucose by stimulating postprandial insulin release from the pancreas, by inhibiting glucagon secretion, and by delaying gastric emptying.17 The 2 main incretins are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP). A defect in the level and function of either incretin has been reported in patients with type 2 diabetes.18 In these patients, there is a reduction in the secretion of GLP-1 and a reduction in the pancreatic response to GIP. Fortunately, the insulinotropic response to GLP-1 is preserved, making modulation of GLP-1 activity a potential therapeutic target.18
DPP-IV, a serine protease widely expressed in many tissues, rapidly inactivates GLP-1 by cleaving its N-terminal amino acids. As a result, the t1/2 of GLP-1 is <2 minutes, limiting its therapeutic utility. Alogliptin is a selective inhibitor of DPP-IV (>10,000-fold greater selectivity versus closely related serine proteases).10 Alogliptin enhances endogenous GLP-1 activity by preventing inactivation of the hormone and therefore prolonging its t1/2. Investigators have demonstrated that once-daily treatment with alogliptin doses ranging from 25 to 400 mg inhibit the activity of DPP-IV by >80% at 24 hours.19
PHARMACOKINETICS
The pharmacokinetic parameters of alogliptin have been evaluated in healthy male volunteers, in adult patients with type 2 diabetes, and in patients with renal or hepatic impairment.
In one trial, single increasing alogliptin doses ranging from 25 to 800 mg were administered to healthy male volunteers (N=36).20 Alogliptin was rapidly absorbed, with a median time to maximum concentration (Tmax) of 1 to 2 hours; dose-dependent increase in drug exposure was observed (mean area under the curve [AUC]0–8, 1,327–49,595 ng h/mL). The mean t1/2 ranged from 12.4 hours in the 800-mg group to 21.4 hours in the 25-mg group. The kidney was the primary route of elimination, with 60% to 71% of drug excreted unchanged in urine.
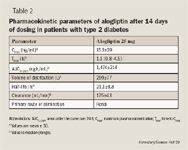
The results of a single-dose (50 mg) pharmacokinetic study in patients with renal impairment demonstrated an increase in alogliptin exposure compared with healthy volunteers.21 Increases in exposure to alogliptin were approximately 1.7-fold in patients with mild renal impairment, approximately 2.1-fold in patients with moderate renal impairment, approximately 3.2-fold in patients with severe renal impairment, and approximately 3.8-fold in patients with end-stage renal disease. Changes in alogliptin pharmacokinetic parameters in patients with moderate hepatic impairment were not clinically significant.22
CLINICAL TRIALS
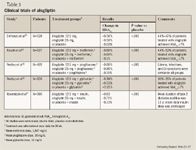
Alogliptin monotherapy. In one trial, the efficacy of alogliptin as monotherapy was evaluated in patients with type 2 diabetes inadequately controlled with diet and exercise.23 Patients (N=329; mean baseline HbA1c, 7.9%) were randomized in a double-blind fashion to receive alogliptin 12.5 mg (n=133), alogliptin 25 mg (n=131), or placebo (n=65) administered once daily for 26 weeks. The mean changes in HbA1c from baseline (the primary end point) were –0.56%, –0.59%, and –0.02% for alogliptin 12.5 mg, alogliptin 25 mg, and placebo, respectively (P<.001 for both alogliptin doses vs placebo). No association was observed between mean changes in HbA1c and age (<65 or ≥65 y), body mass index (BMI) (<30 or ≥30 kg/m2), or race (Hispanic or non-Hispanic). However, mean changes in HbA1c were greater in patients with HbA1c ≥8% at baseline. A total of 47% and 44% of patients treated with alogliptin 12.5 and 25 mg, respectively, achieved HbA1c ≤7% at Week 26 compared with only 23% of patients treated with placebo (P=.001 and P=.008 vs placebo, respectively).

Alogliptin plus pioglitazone. Pratley et al25 reported similar results from a study that evaluated the efficacy and safety of adding alogliptin to pioglitazone in patients with type 2 diabetes inadequately controlled with a thiazolidinedione alone or with a thiazolidinedione plus metformin or a sulfonylurea. A total of 493 patients (mean baseline HbA1c, 8%) were enrolled in this 26-week study. Patients were randomized to receive alogliptin 12.5 mg (n=197), alogliptin 25 mg (n=199), or placebo (n=97) in addition to pioglitazone (mean dose, 35 mg/d). Patients were allowed to remain on their baseline therapy throughout the study. The addition of alogliptin to pioglitazone resulted in improved glycemic control (mean change in HbA1c: alogliptin 12.5 mg, –0.66%; alogliptin 25 mg, –0.80%; placebo, –0.19%; P<.001 for both alogliptin doses vs placebo) regardless of background therapy. The investigators did not report the percentage of patients achieving HbA1c ≤7%. The tolerability and safety results were similar to those reported in the alogliptin plus metformin study, with no significant difference in weight and no significant difference in the incidence of hypoglycemia, edema, infection rates, or GI symptoms among the 3 treatment arms.
Alogliptin plus glyburide. Pratley et al26 conducted another phase 3 trial in patients with type 2 diabetes inadequately controlled with sulfonylurea monotherapy (N=500; mean baseline HbA1c, 8.1%). Patients were randomized to treatment with alogliptin 12.5 mg (n=203), alogliptin 25 mg (n=198), or placebo (n=99) in addition to glyburide (mean dose, 12 mg/d) for 26 weeks. Patients randomized to alogliptin plus glyburide achieved significantly improved glycemic control versus those randomized to placebo plus glyburide (mean change in HbA1c: alogliptin 12.5 mg, –0.38%; alogliptin 25 mg, –0.52%; placebo, +0.01%; P<.001 for both alogliptin doses vs placebo). A total of 30% of patients randomized to receive alogliptin 12.5 mg plus glyburide and 35% of patients who received alogliptin 25 mg plus glyburide achieved HbA1c ≤7% versus 18% of patients who received placebo plus glyburide (P=.057 and P=.002 vs placebo, respectively). There was a slight increase in the rate of hypoglycemia in patients who received alogliptin 12.5 mg (15.8%) compared with patients who received alogliptin 25 mg (9.6%) and those who received placebo (11.1%).
Alogliptin plus insulin. Another study assessed the effect of adding alogliptin 12.5 mg (n=131) or 25 mg (n=129) versus placebo (n=130) to the treatment regimen of patients whose type 2 diabetes was inadequately controlled with insulin alone or with insulin plus metformin.27 Both mean baseline HbA1c (9.3%) and mean duration of type 2 diabetes (13 y) were higher in this study compared with previously mentioned clinical trials. At the end of the 26-week study, mean changes from baseline HbA1c were significantly greater in patients treated with alogliptin 12.5 or 25 mg versus patients treated with placebo (alogliptin 12.5 mg, –0.63%; alogliptin 25 mg, –0.71%; placebo, –0.13%; P<.001 for both alogliptin doses vs placebo). The investigators did not report the proportion of patients achieving HbA1c ≤7% in this study. The combination of alogliptin plus insulin with or without metformin was well tolerated, with no significant difference in weight gain or hypoglycemia among the treatment arms.
DRUG INTERACTIONS
Co-administration of alogliptin with pioglitazone, glyburide, or metformin in healthy volunteers resulted in no significant drug-drug interactions.28–30 Dosage adjustments in patients receiving alogliptin concomitantly with any of these agents is therefore not required. The pharmacokinetic profile of alogliptin administered concomitantly with the CYP450 inhibitors ketoconazole, fluconazole, and gemfibrozil has also been evaluated.31 As expected based on the pharmacokinetic profile of alogliptin, no drug interactions were identified. Alogliptin also demonstrated no effect on the pharmacokinetic or pharmacodynamic profile of warfarin administered once daily in a 7-day study in healthy volunteers.32
ADVERSE EVENTS
In clinical trials, alogliptin generally has been well tolerated across a variety of doses. No dose-limiting toxicities were identified in 2 published trials, and no patients withdrew from these studies because of adverse events.19,20 The proportion of patients in these trials who experienced adverse events was similar for patients receiving various doses of alogliptin (25– 800 mg) and those receiving placebo. Adverse events observed in the trials were classified as mild in nature. The most common adverse events were asymptomatic hypoglycemia (plasma glucose ≤60 mg/dL), headache, dizziness, and constipation. No clinically significant changes in clinical laboratory assessments, vital signs, physical examination, or 12-lead electrocardiogram recordings were reported. Alogliptin was also well tolerated in the pivotal phase 3 studies discussed previously; the incidence of hypoglycemia, GI symptoms, and weight change from baseline was similar in alogliptin-and placebo-treated patients.19,20,23–27
DPP-IV is expressed in many tissues, including T cells, and its inhibition could adversely affect many organ systems. Although the clinical experience with this class of drugs is somewhat limited, immune-related and other serious adverse events have not been observed in clinical trials even with less selective DPP-IV inhibitors and are not expected based on preclinical data.33
Alogliptin has demonstrated an excellent safety profile in clinical trials, but given recent safety concerns observed with other antidiabetic medications, the long-term safety of alogliptin needs to be established.34
DOSING AND ADMINISTRATION
Alogliptin has not received marketing approval by FDA. If approved, this agent will likely be indicated for use with diet and exercise to improve glycemic control in adult patients with type 2 diabetes as monotherapy or in combination with metformin, pioglitazone, or glyburide when treatment with any of these drugs alone provides inadequate glucose control. The NDA is supported by 6 phase 3 studies that evaluated alogliptin doses of 12.5 and 25 mg orally once daily. Alogliptin was well tolerated in older patients enrolled in these studies, so dosage adjustment is not necessary in geriatric patients.
Dosage adjustment is not likely to be required for patients with hepatic insufficiency.22 Systemic exposure of alogliptin has been demonstrated to be increased in patients with renal insufficiency treated with a single 50-mg dose.21 Based on the results of this study, dose adjustment is not likely to be required in patients with mild renal dysfunction. However, the dose should be reduced by 50% and 75% in patients with moderate or severe renal insufficiency, respectively.21 At the time of publication, there were no data available on the safety of alogliptin in pregnant women, nursing mothers, or pediatric patients.
Dr Glode is a PGY2 Oncology Specialty Resident, Roswell Park Cancer Institute, Buffalo, New York. Dr Abdelghany is the coordinator of Investigational Drug Service, Yale New Haven Hospital, New Haven, Connecticut.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
REFERENCES
1. World Health Organization. The diabetes fact sheet (fact sheet no. 312). Available at: http://www.who.int/mediacentre/factsheets/fs312/en. September 2006/. Accessed August 15, 2008.
2. Centers for Disease Control and Prevention. National diabetes fact sheet, 2007. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Accessed August 15, 2008.
3. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986.
4. Reichard P, Nilsson B-Y, Rosenqvist U. The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med. 1993;329:304–309.
5. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [erratum in Lancet. 1999;354:602]. Lancet. 1998;352:837–853.
6. Stettler C, Allemann S, Juni P, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: Meta-analysis of randomized trials. Am Heart J. 2006; 152:27–38.
7. Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes [erratum in Diabetes Care. 2006;49:2816–2818]. Diabetes Care. 2006;29: 1963–1972.
8. American Diabetes Association. Standards of medical care in diabetes-2008. Diabetes Care. 2008;31:S12–S54.
9. Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–342.
10. Deacon CF. Alogliptin, a potent and selective dipeptidyl peptidase-IV inhibitor for the treatment of type 2 diabetes. Curr Opin Investig Drugs. 2008;9:402–413.
11. Januvia [package insert]. Whitehouse Station, NJ: Merck & Co; 2008.
12. Barnett A. DPP-4 inhibitors and their potential role in the management of type 2 diabetes. Int J Clin Pract. 2006;60:1454–1470.
13. Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:376–386.
14 Moritoh Y, Takeuchi K, Asakawa T, et al. Chronic administration of alogliptin, a novel, potent, and highly selective dipeptidyl peptidase-4 inhibitor, improves glycemic control and beta-cell function in obese diabetic ob/ob mice. Eur J Pharmacol. 2008;588: 325–332.
15. Aaboe K, Krarup T, Madsbad S, et al. GLP-1: physiological effects and potential therapeutic applications. Diabetes Obes Metab. 2008 [Epub ahead of print].
16. Feng J, Zhang Z, Wallace MB, et al. Discovery of alogliptin: A potent, selective, bioavailable, and efficacious inhibitor of dipeptidyl peptidase IV. J Med Chem. 2007; 50:2297–2300.
17. Chahal H, Chowdhury TA. Gliptins: A new class of oral hypoglycaemic agent. QJM. 2007; 100:671–677.
18. Idris I, Donnelly R. Dipeptidyl peptidase-IV inhibitors: A major new class of oral antidiabetic drug. Diabetes Obes Metab. 2007;9: 153–165.
19. Covington P, Christopher R, Davenport M, et al. Pharmacokinetic, pharmacodynamic, and tolerability profiles of the dipeptidyl peptidase-4 inhibitor alogliptin: A randomized, double-blind, placebo-controlled, multiple-dose study in adult patients with type 2 diabetes. Clin Ther. 2008;30:499–512.
20. Christopher R, Covington P, Davenport M, et al. Pharmacokinetics, pharmacodynamics, and tolerability of single increasing doses of the dipeptidyl peptidase-4 inhibitor alogliptin in healthy male subjects. Clin Ther. 2008;30:513–527.
21. Karim A, Fleck P, Hetman L, et al. Single-dose pharmacokinetics of the dipeptidyl peptidase-4 inhibitor alogliptin in subjects with renal impairment [abstract]. Presented at: American Diabetes Association 68th Annual Scientific Sessions; June 6–10, 2008; San Francisco, CA. Abstract 538-P.
22. Karim A, Fleck P, Dorsey D, et al. Single-dose pharmacokinetics of alogliptin benzoate (SYR-322), a highly selective dipeptidyl peptidase-4 inhibitor, in subjects with moderate hepatic impairment [abstract]. Presented at: American College of Clinical Pharmacology 36th Annual Meeting; September 9-11, 2007; San Francisco, CA. Abstract 107.
23. Defronzo R, Fleck P, Wilson C, et al. Alogliptin monotherapy improves glycemic control in patients with type 2 diabetes [abstract]. Presented at: American Diabetes Association 68th Annual Scientific Sessions; June 6–10, 2008; San Francisco, CA. Abstract 446-P.
24. Nauck M, Ellis G, Fleck P, et al. Efficacy and safety of alogliptin added to metformin therapy in patients with type 2 diabetes [abstract]. Presented at: American Diabetes Association 68th Annual Scientific Sessions; June 6–10, 2008; San Francisco, CA. Abstract 477-P.
25. Pratley R, Reusch J, Fleck P, et al. Efficacy and safety of alogliptin added to pioglitazone therapy in patients with type 2 diabetes [abstract]. Presented at: American Diabetes Association 68th Annual Scientific Sessions; June 6–10, 2008; San Francisco, CA. Abstract 478-P.
26. Pratley R, Kipnes M, Fleck P, et al. Alogliptin added to sulfonylurea therapy in patients with type 2 diabetes reduces HbA1c without increasing hypoglycemia [abstract]. Presented at: American Diabetes Association 68th Annual Scientific Sessions; June 6–10, 2008; San Francisco, CA. Abstract 445-P.
27. Rosenstock J, Rendel M, Gross J, et al. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA1c without increasing weight gain or hypoglycemia [abstract]. Presented at: American Diabetes Association 68th Annual Scientific Sessions; June 6–10, 2008; San Francisco, CA. Abstract 444-P.
28. Karim A, Fleck P, Joseph M, et al. No pharmacokinetic interaction between alogliptin benzoate (SYR-322) and pioglitazone hydrochloride [abstract]. Presented at: American Diabetes Association 67th Annual Scientific Sessions; June 22–26, 2007; Chicago, IL. Abstract 2117-PO.
29. Fleck P, Karim A, Harris S, et al. Lack of effect of multiple doses of alogliptin benzoate (SYR-322) on the pharmacokinetics of glyburide in healthy subjects [abstract]. Presented at: American Diabetes Association 67th Annual Scientific Sessions; June 22–26, 2007; Chicago, IL. Abstract 2135-PO.
30. Covington P, Christopher R, Davenport M, et al. Lack of pharmacokinetic interaction between alogliptin benzoate (SYR-322) and metformin in healthy subjects [abstract]. Presented at: American Diabetes Association 67th Annual Scientific Sessions; June 22–26, 2007; Chicago, IL. Abstract 2136-PO.
31. Karim A, Fleck P, Harris S, et al. Effect of fluconazole, ketoconazole, and gemfibrozil on the pharmacokinetics of alogliptin benzoate (SYR-322) in healthy subjects [abstract]. Presented at: American College of Clinical Pharmacology 36th Annual Meeting; September 9–11, 2007, San Francisco, CA. Abstract 105.
32. Karim A, Harris S, Fleck P, et al. Assessment of drug interaction between alogliptin benzoate (SYR-322), a highly selective dipeptidyl peptidase-4 inhibitor, and warfarin at steady state [abstract]. Presented at: American College of Clinical Pharmacology 36th Annual Meeting; September 9–11, 2007; San Francisco, CA. Abstract 106.
33. Lankas GR, Leiting B, Roy RS, et al. Dipeptidyl peptidase IV inhibition for the treatment of type 2 diabetes: Potential importance of selectivity over dipeptidyl peptidases 8 and 9. Diabetes. 2005;54: 2988–2994.
34. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes [erratum in N Engl J Med. 2007;357:100]. N Engl J Med. 2007;356:2457–2471.
