- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Alvimopan: A peripherally selective opioid mu receptor antagonist
Post-operative ileus causes significant patient morbidity and is a major contributor to patient discomfort and increased length of hospitalization post-operatively. Alvimopan (Entereg, Adolor/GlaxoSmithKline), a peripherally selective opioid mu receptor antagonist with gastrointestinal (GI) tract-specific activity, is undergoing FDA review for the treatment of post-operative ileus.
Abstract Post-operative ileus causes significant patient morbidity and is a major contributor to patient discomfort and increased length of hospitalization post-operatively. Alvimopan (Entereg, Adolor/GlaxoSmithKline), a peripherally selective opioid mu receptor antagonist with gastrointestinal (GI) tract-specific activity, is undergoing FDA review for the treatment of post-operative ileus. There are currently no other drugs marketed for this indication. In clinical trials, alvimopan was effective in decreasing the time to return of normal GI function after major abdominal surgery without antagonizing the analgesic effect of a systemically administered opioid. Alvimopan appears to be safe and well tolerated when administered for a short duration of therapy up to 7 days after surgery. To date, the only significant interaction documented with alvimopan is the precipitation of local GI adverse effects in chronic opioid users, possibly indicating a localized opioid withdrawal. Alvimopan is administered orally at least 2 hours before a surgical procedure and twice daily starting on Day 1 post-surgery. Further clinical experience will dictate the full utility of alvimopan in the management of post-operative ileus and other dysmotility conditions of the GI tract. (Formulary. 2005;40:176-183.)
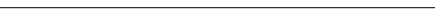
The administration of opioids for the treatment of severe post-operative or chronic pain is associated with the development of bowel dysfunction that can often be dose limiting and debilitating. Opioid-induced bowel dysfunction (OIBD) is characterized by abdominal distention; pain; and constipation and can lead to nausea and vomiting; accumulation of gas and secretions; retention of gastrointestinal contents; impaired absorption of drugs; and fecal impaction.1,2 In the post-operative setting, the use of opioids contributes to and exacerbates post-operative ileus (POI), often resulting in significant discomfort, delay of enteral nutrition, prolonged hospitalization, increased costs, and increase in morbidity. In 1986, the annual cost of post-operative ileus was reported to be $750 million.1
Alvimopan presents advantages in that it has both low systemic absorption and limited ability to enter the central nervous system (CNS). The benefit of this agent lies in its ability to antagonize opioid-mediated changes in gastrointestinal motility without altering the centrally mediated opioid effects, such as analgesia. This article will review alvimopan's pharmacology, pharmacokinetics, clinical trials, safety profile, drug interactions, and dosage and administration. Alvimopan's potential place on the formulary will also be discussed.
CHEMISTRY AND PHARMACOLOGY Alvimopan is a peripherally restricted trans-3,4-dimethyl-4-(3-hydroxyphenyl) piperidine mu opioid receptor antagonist.4,5 It is a small, totally synthetic molecule (C25H32N2O4·2H2O) with a molecular weight of 460.58 g/mol. Alvimopan acts specifically at opioid receptors and has no significant affinity for non-opioid receptors such as adrenergic, dopaminergic, histaminergic, GABAergic, or cholinergic receptors. It has a high affinity for mu opioid receptors (Ki=0.4–0.8 nmol/L) and no significant affinity for the delta (Ki=4.4–7.8 nmol/L) and kappa1, kappa2, or kappa3 opioid receptors (Ki=40–4,050 nmol/L). Restriction to the periphery is due to alvimopan's moderately large molecular weight, zwitterionic form, and polarity. This also prevents passage through the blood-brain barrier and therefore prevents antagonism of analgesic activity. Peripheral restriction has been confirmed in human studies demonstrating a lack of central effect on morphine analgesia and pupil constriction.6
In mouse models, alvimopan was 127 times more selective at the peripheral than at the central mu receptors. Studies using radiolabeled alvimopan indicate that activity is limited to the gastrointestinal (GI) tract. In an in vitro guinea pig ileum assay, alvimopan demonstrated dose-dependent, competitive antagonism of morphine-induced delayed transit of charcoal meal. There were no measurable opioid agonist effects at the mu receptor of the guinea pig ileum or at the delta receptor of the mouse vas deferens. Doses up to 200 mg/kg have no inhibitory or prokinetic effects on the mouse GI tract.
PHARMACOKINETICS Bioavailability was 0.03% in dog models, and preliminary clinical studies in humans have shown that the small amount absorbed is rapidly cleared and does not cross the blood brain barrier.4,5 Plasma concentrations after oral administration are undetectable at doses of 18 mg or less. After oral administration in rats, the vast majority of the alvimopan dose remains in the GI tract, progressively moving from the upper to lower GI tract, and is excreted in the feces within 24 hours.2
Single-dose studies in healthy patients receiving opioid therapy for chronic pain or methadone for opioid addiction demonstrated dose-related increases in the incidence of effective bowel movements and increase in stool weight within 12 hours of alvimopan dosing.2 Maximum response was seen within 4 hours at the 3-mg dose and within 7 hours at the 0.5-mg dose. In a forced titration study in the same population, mean stool weight increased each day until Day 3, with a slight reduction occurring on Day 4 and thereafter. This plateau effect was attributed to elimination of retained feces on Days 1–3 and subsequent return to more normal stool volume by Day 4. In phase 2 trials examining alvimopan's use for prevention of POI, providing the initial dose at least 90 minutes prior to surgery was associated with increased efficacy.
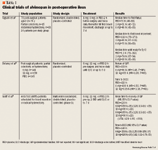
In a randomized, double-blind, placebo-controlled trial, Taguchi et al evaluated the effect of alvimopan on post-operative ileus.7 Seventy-eight patients aged 18 to 78 years scheduled for partial colectomy (n=15) or total abdominal hysterectomy (N=63) were included in the study. Patients were randomly assigned to 1 of 3 treatment groups on the day of surgery: 1 or 6 mg of alvimopan or placebo. There were 26 patients in each group and treatment was given orally with water 2 hours before surgery and twice daily after surgery until the first bowel movement or discharge. Treatment was continued up to post-operative Day 7 in patients with prolonged ileus. Post-operative pain was managed with patient-controlled intravenous morphine or meperidine. Activity and diet were allowed as tolerated by the patient. The time to passage of first flatus, the time to first bowel movement, and the time until the patient was ready for discharge were the primary outcome measures.

Patient ratings of their nausea and abdominal cramping, pain, and itching on a 100 mm visual-analogue scale (VAS) were documented, as well as total daily opioid analgesic use and duration of post-operative hospitalization. The time to ingestion of the first liquid or first solid; time until actual discharge; and patients' VAS rating of nausea and abdominal cramping, itching, and pain were secondary outcomes. Based on intent-to-treat analysis, data from 78 out of 79 patients (26 patients per treatment group) were analyzed. The log-rank test revealed a statistically significant difference in median time to event in the 6-mg alvimopan group when compared with placebo. The median time to passage of first flatus decreased from 70 to 49 hours (P=.03), time to first bowel movement decreased from 111 to 70 hours (P=.01), and time until patients were ready for discharge decreased from 91 to 68 hours (P=.03). The Kruskal-Wallis test revealed that the maximum VAS scores were similar in all 3 groups for pain, itching, and abdominal cramping. Decrease in opioid use as a function of post-operative time and pain score was similar in the 3 groups, demonstrating that alvimopan did not antagonize the analgesic efficacy of systemically administered opioids. The investigators concluded that 6 mg of alvimopan speeds up the recovery of bowel function post-operatively without inhibiting the efficacy of systemic opioids.
In study 14CL302, Delaney et al showed that alvimopan accelerated the recovery of gastrointestinal function after major abdominal surgery.8 The investigators conducted a prospective, multicenter, double-blind, placebo-controlled trial of 424 patients undergoing partial colectomy or simple or radical hysterectomy. Patients were randomized to receive 6 mg of alvimopan (n=141), 12 mg of alvimopan (n=138), or placebo (n=145) orally 2 hours before surgery and twice daily thereafter until discharge or for up to a maximum of 7 days. Time to return to gastrointestinal function (GIF), defined as passage of flatus or stool and tolerating solid food, was the primary efficacy end point. Time to writing of hospital discharge order (DCO) was the secondary end point. The Cox proportional hazard model and Wald Chi2 test showed that time to GIF recovery and DCO were significantly accelerated in patients who received 6 mg of alvimopan compared to placebo, and little effect was seen with 12 mg of alvimopan. Nausea, vomiting, and hypotension were the most common adverse effects in the whole study group but the alvimopan group had a lower incidence of nausea and vomiting. The investigators concluded that alvimopan accelerated recovery of gastrointestinal function and time to hospital discharge and was well tolerated.
The efficacy and safety of alvimopan was also studied in another phase 3 multicenter, randomized, double-blind, placebo-controlled trial.9 Alvimopan 6 or 12 mg or placebo were randomly assigned to 510 patients scheduled for bowel resection or radical hysterectomy. Patients had to be aged at least 18 years for study inclusion. The treatment medications were given at least 2 hours before surgery, then twice daily starting on post-operative Day 1 until hospital discharge up to a maximum of 7 days post-surgery. The primary efficacy end point was the time to recovery of GI function (GI-3) and was defined by the later of 2 events: the time the patient tolerated solid food and the time the patient passed either flatus or a bowel movement (BM). The secondary end point (GI-2) was defined by the later of 2 events: the time the patient tolerated solid food or the time the patient passed the first BM. Need for post-operative nasogastric tube (NGT) insertion, severity of GI symptoms, opioid consumption, and pain control with VAS were also monitored. Patients were evaluated for adverse events for up to 30 days after discharge. Treatment effect on all time-to-event data was assessed using the Cox proportional hazard model, and the Wald Chi2 test was used to calculate P values for comparisons of alvimopan 6 and 12 mg versus placebo.
Data from 469 out of 510 patients were included in the analysis based on modified intent-to-treat (MITT) analysis. Time to recovery of GI function (GI-3) was significantly accelerated by alvimopan at the 6-mg dose (HR=1.28; 95% CI, 1.00–1.64; P<.05) and 12-mg dose (HR=1.54; 95% CI, 1.21-1.96; P<.001). This translated to a mean time decrease for the return of GI function (GI-1) of approximately 15 and 22 hours for 6- and 12-mg alvimopan groups, respectively, as analyzed by Kaplan-Meier cumulative survival curve. When compared to placebo for GI-2, the mean time that patients first tolerated solid food or passed first BM was accelerated by approximately 20 hours in the 6-mg group and 28 hours in the 12-mg group. Time to hospital discharge order was approximately 13 and 20 hours earlier for the alvimopan 6-mg and 12-mg groups, respectively. Need for NGT insertion was less in the alvimopan group and reached statistical significance in patients receiving the 12-mg dose (P=.004). Treatment-associated adverse effects were similar for all the groups with a lower incidence of nausea and vomiting in the alvimopan groups. Average daily opioid use was comparable for the alvimopan 12-mg group (27.7 mg) and the placebo group (27 mg) but was slightly higher for the alvimopan 6-mg group (33.6 mg). The investigators concluded that alvimopan 6 or 12 mg twice daily accelerated recovery of GI function after laparotomy for bowel resection or radical hysterectomy.
ADVERSE EFFECTS Alvimopan is generally well tolerated and does not appear to reverse opioid analgesia at therapeutic doses. In all clinical trials of patients receiving post-operative opioid therapy, treatment-emergent adverse events were comparable between alvimopan-treated patients and patients receiving placebo, with the exception of a greater incidence of nausea, vomiting, and post-operative ileus occurring in the placebo group. However, in patients receiving chronic opioid therapy, the use of alvimopan may precipitate a dose-related, localized, gut-specific withdrawal.
A phase 1 double-blind, placebo-controlled, dose-ascending study evaluated the safety and dose-related side effects of alvimopan at doses up to 54 mg daily in 44 healthy volunteers.2 Patients were randomized to 0.25, 0.5, 2.0, 6.0, or 18.0 mg three times daily. The most commonly observed adverse events were dose-related and included abdominal pain (31%), flatulence (31%), and diarrhea (21%). Nausea, polyuria, and nervousness were also reported. All side effects were dose-related and occurred infrequently at lower doses. Elevations in liver function tests (aspartate aminotransferase, alanine aminotransferase, and lactic dehydrogenase) from 2.5 to 4 times the upper limit of normal occurred in 2 patients, but no dose-related hepatotoxicity was reported.
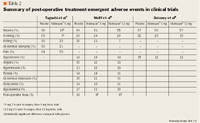
The most commonly observed treatment-emergent adverse events occurring in the Wolff (phase 3) and Delaney trials were nausea, vomiting, and hypotension. Nausea and vomiting occurred in a slightly higer, but not statistically significant, percentage of patients in the placebo group than in the alvimopan 6-mg group, and there was no difference between the groups in incidence of hypotension. In the Wolff trial, serious adverse events considered by the investigators to be related to the study drug were experienced by 1.2% of patients in the placebo group and 3% of patients in the alvimopan 6-mg group. The percentage of patients who discontinued treatment because of adverse events in the Wolff trial was 2.4% for the alvimopan 6-mg group and 4.2% for the placebo group. In the Taguchi trial, nausea and vomiting occurred significantly less frequently in the alvimopan 6-mg group. There were no adverse events that were considered to be related to the administration of alvimopan.

In pre-clinical studies in animals, oral administration of alvimopan to rats up to 200 mg/kg and to dogs up to 100 mg/kg revealed no important toxicologic findings. Evaluation of alvimopan in preliminary in vitro and in vivo genetic toxicology tests in animals also revealed no positive findings.4
PHARMACOKINETIC AND PHARMACODYNAMIC INTERACTIONS At this time no drug-drug or food-drug interaction studies have been performed. Although human absorption data are not available, the degree of absorption in human subjects would be expected to be very low based on animal studies. Systemic pharmacokinetic interactions would, therefore, also be expected to have minimal clinical significance.
Interactions at the site of action in the gut, although not studied, may be a consideration. It is unknown whether the presence of food or high concentrations of mu receptor agonists impact the activity of alvimopan locally in the gastrointestinal tract.
As mentioned previously, patients on chronic opioid therapy who were administered alvimopan have experienced a reversal of local gastrointestinal opioid effects characterized by abdominal cramping and gas, possibly indicating a blocking of opioid agonist activity at the local mu receptors and a gut-specific opioid withdrawal effect.10
In phase 3 clinical trials, daily and maximum post-operative pain scores were comparable among groups receiving alvimopan and placebo, thereby indicating that antagonism of opioid analgesia by alvimopan was not clinically significant.9
DOSAGE AND ADMINISTRATION Alvimopan at doses of 6 or 12 mg 2 hours prior to surgery, followed by twice daily administration thereafter, has induced accelerated recovery of gastrointestinal function in clinical studies of patients undergoing bowel resection or radical hysterectomy. No clinically evident antagonism of opioid analgesia has occurred at these doses.9 The 6-mg and 12-mg doses have also been shown to decrease time to discharge order by 13 hours and 20 hours, respectively, compared with placebo. Since it is minimally absorbed, no dosage adjustment is expected to be required for age, renal impairment, or hepatic impairment.5
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, director of Drug Information Services at Hartford Hospital in Hartford, Conn, and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn; and by Craig I. Coleman, PharmD, assistant professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
EDITORS' NOTE: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
Dr Udeh is clinical coordinator, surgery, at Hartford Hospital, Hartford, Conn, and adjunct clinical professor at the University of Connecticut School of Pharmacy, Storrs, Conn. She can be reached at eudeh@harthosp.org
. Dr Goldman is a drug information specialist at Hartford Hospital and adjunct clinical professor at the University of Connecticut School of Pharmacy.
REFERENCES 1. Kurz A, Sessler DI. Opioid-induced bowel dysfunction. Drugs. 2003;63(7):649–671.
2. Schmidt WK. Alvimopan (ADL-8-2698) is a novel peripheral opioid antagonist. Am J Surg. 2001;182(Suppl to November 2001):27S–38S.
3. Adolor Corporation news releases. Adolor completes submission of NDA for Entereg (alvimopan) in postoperative ileus. Available at: http://phx.corporate-ir.net/phoenix.zhtml?c=120919&p=irol-newsArticle&t=Regular&id=585562&. Accessed May 11, 2005.
4. Zimmerman DM, Gidda JS, Cantrell BE, et al. LY246736 Dihydrate. Mu opioid receptor antagonist. Drugs Future. 1994;19:1078–1083.
5. Zimmerman DM, Gidda JS, Cantrell BE, et al. Discovery of a potent, peripherally selective trans-3,4-dimethyl-4-(3-hydroxyphenyl) piperidine opioid antagonist for the treatment of gastrointestinal motility disorders. J Med Chem. 1994;37: 2262–2265.
6. Liu SS, Hodgeson PS, Carpenter RL, Fricke JR. ADL 8-2698, a trans-3,4-dimethyl-4-(3-hydroxyphenyl) piperidine, prevents gastrointestinal effects of intravenous morphine without affecting analgesia. Clin Pharmacol Ther. 2001;69(1): 66–71.
7. Taguchi A, Sharma W, Saleem RM, et.al. Selective postoperative inhibition of gastrointestinal opioid receptors. N Engl J Med. 2001;345(13): 935–940.
8. Delaney C, Weese J, Hyman N. Prospective, randomized, double-blind, multicenter, placebo-controlled study of alvimopan, a novel peripherally-acting mu opioid antagonist for postoperative ileus after major abdominal surgery [abstract]. Dis Colon Rectum. 2004;47:589.
9. Wolff BG, Michelassi F, Gerkin TM, et al. Alvimopan, a novel peripherally acting mu opioid antagonist; results of a multicenter, randomized, double-blind, placebo-controlled, Phase III trial of major abdominal surgery and postoperative ileus. Ann Surg. 2004;240(4):728–735.
10. Liberto JG, Herschler JA, Jaffe JH, et al. Effects of ADL 8-2698, a peripherally restricted mu opioid antagonist, on gut mobility in methadone and LAAM dependent patients with opioid-induced constipation: a dose ranging study [abstract]. Drug Alcohol Depend. 2001:63:S91.
11. Paulson DM, Kennedy DT, Donovick RA, et al. An oral, peripherally acting, mu opioid receptor antagonist for the treatment of opioid-induced bowel dysfunction for the treatment of opioid induced bowel dysfunction-a 21-day treatment-randomized clinical trial. J Pain. 2004:6(3): 184–192.
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
