- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
American College of Cardiology: 55th Annual Scientific Session focuses on continuing research, emerging clinical trial data
The 55th Annual Scientific Session of the American College of Cardiology (ACC) assembled from March 11 to March 14, 2006, in Atlanta, Ga, to exchange new and continuing research in cardiovascular disease. The program featured more than 1,600 oral and poster presentations of original research and hundreds of invited lectures and interactive sessions, with many offering the opportunity to update attendees' knowledge of available and investigational pharmaceuticals.

This year's program featured several important clinical trials in which antithrombotic therapies were compared in patients with various forms of acute coronary syndromes (ACS). These included ACUITY, ISAR-REACT 2, ExTRACT-TIMI 25, CHARISMA, and OASIS-6. Other trials of interest to formulary decision-makers were ASTEROID, ARMYDA-3, PRIMO-CABG II, and OPTIMIZE-HF.
Following are summaries of each of these trials, with the key end points presented and, when possible, their implications for clinical practice. The data from some of these trials has since been published in primary research journals; where applicable, all citations available at press time are included within each summary.
Bivalirudin alone leads to net clinical benefit in ACS
Bivalirudin with or without glycoprotein (GP) IIb/IIIa inhibitors is as effective as unfractionated heparin (UFH) or enoxparin plus IIb/IIIa inhibition in preventing ischemic complications in patients with ACS, said Gregg W. Stone, MD, lead investigator of the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial.
The bivalirudin-alone strategy was associated with a significant reduction in major bleeding compared with UFH/enoxaparin plus IIb/IIIa inhibition, although it did not result in fewer ischemic events.
In ACUITY, 13,819 patients with moderate- to high-risk ACS who underwent cardiac catheterization within 72 hours were assigned to one of 3 treatment regimens: UFH or enoxaparin plus a GP IIb/IIIa inhibitor (arm A), bivalirudin plus a GP IIb/IIIa inhibitor (arm B), or bivalirudin alone (arm C). About 60% of the study population received clopidogrel pretreatment.

The primary end point was net clinical benefit at 30 days, defined as a composite of adverse ischemic events (death, myocardial infarction (MI), or unplanned revascularization), or major bleeding. The trial was powered for sequential noninferiority and superiority.
Bivalirudin plus a GP IIb/IIIa inhibitor (arm B) was found to be noninferior to UFH/enoxaparin plus a GP IIb/IIIa inhibitor (arm A) on the primary end point and its individual components. The net clinical outcome was achieved by 11.7% of patients in arm A and 11.8% in arm B (P<.001 for noninferiority); the incidence of ischemic events was 7.3% in arm A and 7.7% in arm B (P=.007 for noninferiority), and major bleeding occurred in 5.7% of patients in arm A and 5.3% in arm B (P=.001 for noninferiority).
The net clinical outcome was achieved by 10.1% of patients in arm C, which was superior to arm A (P=.015). This superiority was driven by a significantly lower rate of bleeding in arm C versus arm A (3.0% vs 5.7%; P<.001). The incidence of ischemic events in arm C was 7.8%, which did not achieve superiority over arm A (7.3%), but did meet the test of noninferiority (P=.011).
In patients with elevated creatine kinase/troponin levels, there was no significant benefit to bivalirudin monotherapy compared with arm A. In patients with negative cardiac markers, bivalirudin alone was associated with a 25% reduction in the net clinical end point, and the upper confidence interval did not reach 1.0 (95% CI, 0.61–0.93).
"In patients with moderate- to high-risk ACS undergoing an early invasive strategy with the use of GP IIb/IIIa inhibitors, bivalirudin is an acceptable substitute for either UFH or enoxaparin," said Dr Stone, professor of medicine and director of cardiovascular research and education at Columbia University Medical Center, New York, NY.
"However, compared to either UFH/enoxaparin with GP IIb/IIIa inhibition or bivalirudin with GP IIb/IIIa inhibition, a bivalirudin-alone strategy results in significantly greater net clinical benefit and enhanced survival, free from adverse events at 30 days," Dr Stone stated.
The relevance of ACUITY was questioned by Eric Peterson, MD, MPH, who remarked that 30% of the study population had neither ECG changes nor positive cardiac biomarkers, and 40% had negative cardiac markers. "This is a population we wouldn't anticipate IIb/IIIa inhibitors working in," he said. "Only one-third of the ACUITY population would have qualified for other trials testing an early invasive strategy."
Dr Peterson cited that the event rates in ACUITY were less than those in other trials in which GP IIb/IIIa inhibitors were employed. In addition, 70% of the patients had received some form of antithrombotic therapy between the time they were admitted and the time they were randomized into the study. Therefore, "it doesn't answer how best to treat patients showing up in the emergency room," said Dr Peterson, co-director of cardiovascular research and director of cardiovascular outcomes research and quality at the Duke Clinical Research Institute, Durham, NC.
In the patients pretreated with clopidogrel, bivalirudin monotherapy was associated with a reduction in the net clinical composite outcome compared with UFH/enoxaparin plus a IIb/IIIa inhibitor, whereas there was no benefit to bivalirudin monotherapy in patients who did not receive clopidogrel pretreatment, Dr Stone said.
Shamir Mehta, MD, associate professor of medicine at McMaster University in Hamilton, Ontario, Canada, noted the lack of a reduction in ischemic events with bivalirudin compared with arms A and B, and that the reduction in bleeding occurred only in patients on bivalirudin monotherapy. "It's expected that bleeding will be lower in patients receiving 1 antithrombotic drug compared with 2," Dr Mehta said.
ISAR-REACT 2
Abciximab reduces adverse event risk in ACS patients pretreated with clopidogrel
The GP IIb/IIIa antagonist abciximab reduces the risk of adverse events in patients with ACS who undergo percutaneous coronary intervention (PCI), even if the patients are pretreated with clopidogrel, said Adnan Kastrati, MD.
In the Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment 2 (ISAR-REACT 2) study, the primary end point-a composite of death, MI, and urgent target vessel revascularization-was reduced significantly with abciximab in ACS patients who had received a 600-mg loading dose of clopidogrel. Subgroup analysis showed that patients with positive troponin levels derived clinical benefit from abciximab, whereas those with low levels of troponin had no significant reduction in the incidence of the primary end point, reported Dr Kastrati, from Deutsches Herzzentrum in Munich, Germany.
Pretreatment with a loading dose of clopidogrel has become standard therapy in patients who undergo PCI, Dr Kastrati said.
ISAR-REACT 2 was a randomized, double-blind study that included 2,022 patients with non-ST-segment elevation MI undergoing PCI. All patients received 600 mg clopidogrel at least 2 hours prior to the procedure, as well as 500 mg of oral or intravenous aspirin. They were then randomized to abciximab, given as a bolus of 0.25 mg/kg of body weight followed by a 12-hour infusion at 0.125 mcg/kg/min, or to placebo.
At 30 days after randomization, 8.9% of patients assigned to abciximab and 11.9% assigned to placebo reached the primary end point, a relative risk reduction of 25% (P=.03). There was no reduction in the incidence of the primary end point among patients with troponin levels <0.03 mcg/L (4.6% in each group). In patients with elevated troponin levels, the incidence of the primary end point was 13.1% in those assigned to abciximab compared with 18.3% in those assigned to placebo (29% relative risk reduction; P=.02).
The difference between ISAR-REACT 2 and previous trials of GP IIb/IIIa inhibitors in patients with ACS is that randomization in ISAR-REACT 2 occurred after patients had been diagnosed with coronary artery disease on angiography, ensuring a higher risk profile of patients enrolled, said Dr Kastrati.
Since its presentation, the results of ISAR-REACT 2 have been published in the Journal of the American Medical Association (2006;295:1531–1538).
In an editorial accompanying the article, Steven R. Steinhubl, MD, associate professor and director of cardiovascular education and clinical research, and Richard Charnigo, PhD, assistant professor of biostatistics, both at the University of Kentucky in Lexington, Ky, said: "Given current evidence, all heparin-treated patients undergoing PCI for treatment of ACS with elevated troponin levels should receive adjunctive GP IIb/IIIa antagonists, irrespective of whether the patient has also received adequate pretreatment with clopidogrel. Whether there is additional clinical benefit to administering clopidogrel in addition to a GP IIb/IIIa antagonist, as has been suggested by previous post hoc analysis, remains to be prospectively studied."
ExTRACT-TIMI 25
Enoxaparin demonstrates net clinical benefit in ST-segment elevation MI
Enoxaparin is superior to UFH in preventing death or recurrent MI in patients with ST-segment elevation MI who are treated with fibrinolytic therapy, said Elliott M. Antman, MD, lead investigator of the Enoxaparin and Thrombolysis Reperfusion for Acute Myocardial Infarction Treatment (ExTRACT-TIMI 25) trial.
A reduction in nonfatal MI was responsible for the significant reduction in the primary composite end point, with no effect of enoxaparin on mortality. The favorable effect on death/MI was accompanied by an increase in bleeding episodes, but a measure of net clinical benefit that incorporated clinical end points and bleeding favored the enoxaparin strategy, said Dr Antman, director of the Samuel A. Levine Cardiac Unit at Boston's Brigham and Women's Hospital, and professor of medicine at Harvard Medical School in Boston, Mass.
"A strategy of enoxaparin is clearly preferable to the current standard of UFH as the antithrombin to support fibrinolysis, the most common form of reperfusion for ST-segment elevation MI used worldwide," Dr Antman said.
ExTRACT-TIMI 25 included 20,479 patients who presented within 6 hours of an MI and were eligible for lytic therapy. In a double-blind, double-dummy fashion, patients were randomized to enoxaparin administered throughout the index hospitalization, with the dosage based on age and creatinine clearance, or bolus/infusion of UFH for at least 48 hours.
The regimen of enoxaparin used in the study, with modifications for age and creatinine clearance, was designed specifically to reduce the risk of bleeding, Dr Antman said.
The primary end point-death or nonfatal recurrent MI through 30 days-occurred in 12% of patients randomized to UFH and in 9.9% randomized to enoxaparin, corresponding to a 17% relative reduction in risk with enoxaparin (P<.001).
The benefit was apparent within 48 hours. At 1 month, enoxaparin was associated with a 33% reduction (P<.001) in the risk of recurrent MI compared with UFH. The incidence of death at 30 days was not significantly different between the enoxaparin and UFH groups (6.9% vs 7.5%, respectively; P=.11). The need for urgent revascularization by 30 days also was reduced significantly in the enoxaparin group (26% reduction; P<.001). There was a 53% increase in major bleeding episodes in patients assigned to enoxaparin (P<.001), but no significant increase in the risk of intracerebral hemorrhage.
In commenting on the net clinical benefit with enoxaparin in fibrinolytic-treated patients with ST-segment elevation MI, Dr Antman said that compared with UFH, the enoxaparin strategy would result in 15 fewer nonfatal MIs, 7 fewer episodes of urgent revascularization, and 6 fewer deaths per 1,000 treated patients, but at the expense of 4 episodes of nonfatal major bleeding.
He stated that a superior antithrombotic effect of enoxaparin, a more sustained antithrombotic effect of enoxaparin owing to a longer duration of treatment, and possibly a rebound increase in thrombotic events after discontinuation of UFH may have contributed to enoxaparin superiority in this study. The ExTRACT-TIMI 25 results have since been published in the New England Journal of Medicine (2006;354:1477–1488).
CHARISMA
Dual antiplatelet therapy fails to reduce CV event risk in high-risk patients
Clopidogrel failed to reduce the risk of cardiovascular events in stable aspirin-treated patients at high risk of such events, and the addition of clopidogrel to aspirin in high-risk patients without existing cardiovascular disease increased the risk of vascular events.
The study was noteworthy in that the subgroup of patients with established cardiovascular disease experienced a modest reduction in the risk of adverse cardiovascular events and no increased risk of severe bleeding with dual antiplatelet therapy, whereas the patients with multiple risk factors alone received no benefit and had an increased risk of severe bleeding from the addition of clopidogrel to aspirin, Dr Bhatt said.
"Our hypothesis is that perhaps patients who fall under the secondary prevention umbrella have 'revved up' platelets, and adding clopidogrel to aspirin restores them to a steady state," said Dr Bhatt, director of the Cardiovascular Trials Unit and associate director of the Cleveland Clinic Cardiovascular Coordinating Center, Cleveland, Ohio. "The bleeding risk may be mitigated because their platelets are already revved up, whereas primary prevention patients are exposed to bleeding risk," he said.
CHARISMA included 15,603 patients with either documented cardiovascular disease or multiple risk factors for atherosclerosis who were randomized to placebo or clopidogrel, 75 mg/d, in addition to low-dose aspirin. Patients were followed for a median of 28 months.
In the overall population of patients, the primary end point-the combined rate of cardiovascular death, MI, or stroke-occurred in 7.3% of patients assigned to placebo and 6.8% assigned to clopidogrel, a nonsignificant difference (P=.22). When hospitalization for an ischemic event was added to the primary outcome, clopidogrel reduced the combined risk from 17.9% to 16.7% (P=.04).
The overall outcome was influenced by divergent findings in the 2 main subgroups enrolled in the trial, Dr Bhatt said.
In the patients without qualifying cardiovascular disease, dual therapy was associated with a 20% increase in the risk of cardiovascular death/MI/stroke compared with aspirin alone (P=.20). In the primary prevention cohort, severe bleeding was increased by 67% with the addition of clopidogrel to aspirin (P=.07), and moderate bleeding was increased by 60% (P=.08).
The excess deaths in the primary prevention group were not related to the increase in bleeding but rather an increase in cardiovascular events, Dr Bhatt said. "It didn't appear that the ones who suffered bleeding were the ones who died," he said.
The rate of death from cardiovascular causes in the primary prevention cohort was 3.9% among the clopidogrel recipients and 2.2% in placebo recipients (P=.01).
In the 12,153 patients with established cardiovascular disease, the addition of clopidogrel reduced the occurrence of the primary end point from 7.9% to 6.9%, a relative risk reduction of 12.5% (P=.046).
The secondary prevention patients had no excess in the risk of severe bleeding but did have a 63% increased risk of moderate bleeding (P<.001) compared with aspirin alone.
"We had 12,000 patients in the secondary prevention subgroup, and there was a suggestion of a benefit. It would be a shame to throw the baby out with the bathwater," Dr Bhatt said.
W. Douglas Weaver, MD, chair of cardiology and co-director of the Heart and Vascular Institute at Henry Ford Hospital in Detroit, Mich, disagreed: "The primary end point was negative. I don't think it's even fair to look at secondary end points. Our indications for clopidogrel should not change after this study."
The decision to use clopidogrel in stable patients should be individualized, Dr Bhatt said. "We need to weigh the ischemic risk against the risk of bleeding. For a patient with multiple MIs while on aspirin, I would consider adding clopidogrel," he said. "But I wouldn't add clopidogrel in a patient who had a prior MI and has been stable since and had a bleeding ulcer while on aspirin."
"The bottom line, in primary prevention, is that there is no reason to use clopidogrel based on its lack of efficacy and the excess bleeding seen," Dr Bhatt said.
The data from CHARISMA have since been published in the New England Journal of Medicine (2006;354:1706–1717).
OASIS-6
Fondaparinux reduces event rate in ST-segment elevation MI, except in primary PCI
Fondaparinux reduces the risk of death or recurrent MI compared with standard antithrombotic therapy (UFH or placebo) in patients with ST-segment elevation MI, said Salim Yusuf, DPhil. In a subset of patients undergoing primary PCI, however, fondaparinux was not superior to UFH in reducing death or recurrent MI and was associated with a greater risk of catheter thrombosis.

If UFH was not indicated, patients were randomized to up to 8 days of subcutaneous treatment with fondaparinux, 2.5 mg/d, or placebo. In the group in which UFH was indicated, patients were randomized to fondaparinux, 2.5 mg/d, or UFH. Patients were followed for a minimum of 90 days and a maximum of 180 days.
The primary efficacy outcome-death or MI at 30 days-was reduced by 14% (P=.008) in the fondaparinux versus the control groups (9.7% vs 11.2%, respectively), said Dr Yusuf, the study's principal investigator and professor of medicine, McMaster University and Hamilton Health Sciences, Ontario, Canada. "This advantage of fondaparinux on death/MI was observed at 9 days (P=.003) and sustained to 180 days (P=.008)," he said.
The favorable effect of fondaparinux on the primary end point was apparent in stratum 1 and stratum 2. However, there was no advantage to fondaparinux in patients undergoing primary PCI. The rates of death/MI and bleeding were not significantly different between the fondaparinux and UFH groups in patients undergoing primary PCI, but the incidence of guiding catheter thrombosis and coronary complications (ie, abrupt artery closure, catheter thrombus, new angiographic thrombus) was greater in the fondaparinux group.
"UFH should be used for primary PCI patients as initial therapy. For others, fondaparinux should be given," Dr Yusuf said. "After removing the early hazard in primary PCI patients, there was a reduction in ischemic events and no increase in bleeding with fondaparinux."
Among the patients in stratum 2 not undergoing primary PCI, fondaparinux was associated with an 18% reduction in death/MI at 30 days (P=.08) and a 23% reduction at study's end (P=.008). There was a tendency toward fewer severe and fatal bleeds with fondaparinux (P=.13 for both), and there was significantly less cardiac tamponade (41% relative risk reduction; P=.02).
In looking at death and the net clinical benefit at study's end, fondaparinux significantly reduced death in the overall OASIS-6 population (P=.029) and in those receiving thrombolytic therapy (P=.04); and it was associated with significant reductions in the composite of death/MI/stroke/severe hemorrhage in the overall study population (P=.009), in those not receiving reperfusion therapy (P=.016), and in those receiving thrombolytic therapy (P=.007).
Taken with the results of OASIS-5, which demonstrated that fondaparinux was as effective as enoxaparin in reducing the risk of adverse cardiovascular outcomes over the short-term in patients with ACS, while causing less bleeding, "we showed that fondaparinux benefits a broad spectrum of patients with ACS," Dr Yusuf said. "Similar data are lacking with other antithrombotics, except aspirin."
The ease of use of fondaparinux, which can be given as a single fixed dose, the lack of monitoring required with its use, its low cost, and its efficacy and safety in the full spectrum of patients with ACS facilitate its use in a range of settings, he said.
In response to criticism that primary PCI is the preferred treatment for ST-segment elevation MI in the United States, thus limiting the role of fondaparinux for this patient population, Dr Yusuf said that perhaps 80% of patients with ST-segment elevation MI are not treated with primary PCI, and those who do undergo primary PCI can still benefit from the drug if given several days after PCI.
Findings from the study have since been published in the Journal of the American Medical Association (2006;295:1519–1530).
ASTEROID
Coronary atherosclerosis regression possible with intensive statin therapy

"Until now, the best we've been able to achieve is to slow the progression of disease, and we've actually gotten close to zero progression," Dr Nissen said. "What we haven't been able to achieve is to actually reverse, even partially regress, the disease."
ASTEROID was an open-label, blinded study in which patients with coronary artery disease, defined as >20% stenosis in any major coronary artery, were treated with rosuvastatin 40 mg/d for 24 months. Final IVUS data were available from 349 patients enrolled.
From baseline to 24 months, the mean low-density lipoprotein cholesterol (LDL-C) level was decreased from 130.4 mg/dL to 60.8 mg/dL, "the lowest ever achieved in a major clinical trial in which the effect of statin therapy on atherosclerosis was studied," said Dr Nissen, medical director of The Cleveland Clinic, Cleveland, Ohio. "Also, very surprisingly, we got an increase in the high-density lipoprotein cholesterol of 14.7%, which is really unprecedented." The level of HDL cholesterol (HDL-C) increased from 43.1 mg/dL at baseline to 49.0 mg/dL.
The primary efficacy end points were the percentage of change in atheroma volume and the change in atheroma volume in the 10-mm segment with the greatest disease severity at baseline.
Atheroma volume for the entire examined vessel decreased by a mean of 0.98% and a median of 0.79% from baseline (P<.001), and 63.6% of the study group experienced some regression of their atherosclerosis.
In the most diseased 10-mm segments, atheroma volume regressed by a mean of 6.1 mm3 and a median of 5.6 mm3 (P<.001). This change represents a median reduction of approximately 9% in atheroma volume in the 10-mm segment with the greatest disease severity.
Based on the results of ASTEROID, "maximally intensive statin therapy seems warranted in high-risk patients with CAD," Dr Nissen said.
The results of ASTEROID have since been published in the Journal of the American Medical Association (2006;295:1556–1565).
In an editorial accompanying the publication of the ASTEROID results, Roger S. Blumenthal, MD, and Navin K. Kapur, MD, from The Johns Hopkins Ciccarone Preventive Cardiology Center in Baltimore, Md, said: "While IVUS-documented atherosclerotic regression is an intriguing finding, clinicians must remember that this may not be the best measure of the treatment's effect on hard cardiovascular end points."
At the ACC conference, Dr Nissen remarked that, "we're looking at the disease itself with IVUS. I expect that we'll find that this signal is confirmed with morbidity and mortality benefit."
Session moderator James Stein, MD, co-director of preventive cardiology at the University of Wisconsin, in Madison, Wisc, noted that other clinical trials such as the Familial Atherosclerosis Treatment Study (FATS), the HDL-Atherosclerosis Treatment Study (HATS), and the St. Thomas' Atherosclerosis Regression Study (STARS), have documented regression of coronary atherosclerosis using coronary angiography.
Dr Nissen replied that because these trials used angiography to measure the percentage of stenosis in the most diseased vessel segment, the findings merely reflected a regression to the mean and not regression of atheroma volume.
Despite the success of intensive therapy to reduce LDL-C levels to well below those recommended in current guidelines for the treatment of patients with CAD, "it is by no means sufficient to alter guidelines, and individual physicians will make up their own minds about how compelling an argument can be made for taking LDL lower," Dr Nissen said. "I wonder if the best standard is not to use target cholesterol levels but to say, 'let's get our patients with coronary disease as low an LDL cholesterol as achievable without creating safety problems.' "
ARMYDA-3
Postoperative AF blunted by statin pretreatment
Pretreatment with atorvastatin for 7 days reduced the incidence of new-onset atrial fibrillation (AF) in patients undergoing cardiac surgery in the Atorvastatin for Reduction of Myocardial Dysrhythmia After Cardiac Surgery (ARMYDA-3) study.
"The low cost and low risk of this therapy may support a routine early initiation of atorvastatin treatment in this population of patients," said Germano Di Sciascio, MD, who presented the results of the ARMYDA study to the conference.
AF occurs in up to 40% of patients following coronary artery bypass graft (CABG) surgery and in more than 50% after valve operations, and may lead to prolonged ventilatory and inotropic support and increased hospital stays and costs.
Postcardiac surgery AF may have an underlying inflammatory etiology that might be suppressed by statin therapy, said Dr Di Sciascio, professor and chairman of cardiology and director, department of cardiovascular sciences, Campus Biomedico, University of Rome, Rome, Italy, in explaining the study rationale.
Two hundred patients undergoing elective CABG or heart valve replacement/repair were randomized to placebo or atorvastatin 40 mg/d, initiated 7 days before surgery. The incidence of postoperative in-hospital AF was the primary end point. AF was defined as an arrhythmic episode lasting more than 5 minutes, registered on continuous telemetry or 12-lead electrocardiogram.
Postoperative AF occurred in 35% of patients randomized to atorvastatin compared with 57% of those randomized to placebo (P=.003).
Multivariate analysis showed a 60% reduction in the risk of AF with atorvastatin treatment, Dr Di Sciascio said.
Blood levels of C-reactive protein were significantly higher in patients who developed AF compared with those who did not, irrespective of the group to which patients were assigned, he said.
The duration of hospital stay was 6.3 days and 6.9 days in the atorvastatin and placebo recipients (P=.001), respectively.
PRIMO-CABG II
No reduction in primary end point with pexelizumab
The terminal complement inhibitor pexelizumab did not significantly reduce the incidence of perioperative MI and/or death in high-risk patients undergoing on-pump CABG surgery, said Peter K. Smith, MD.
In the Pexelizumab for the Reduction of Infarction and Mortality in Coronary Artery Bypass Graft Surgery II (PRIMO-CABG II) study, 4,254 patients undergoing on-pump CABG with or without valve surgery who had at least 2 prespecified risk factors were randomized to receive pexelizumab, given as a 2.0-mg/kg bolus followed by an infusion of 0.05 mg/kg per hour for 24 hours, or to placebo immediately prior to surgery.
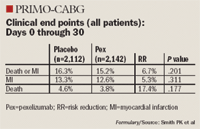
There was a 17.4% reduction in death with pexelizumab, from 4.6% to 3.8%, which again did not achieve significance (P=.177). This trend in mortality persisted, but the study was underpowered to achieve statistical significance for this reduction in death, said Dr Smith, chief of thoracic and cardiovascular surgery, Duke University, Durham, NC.
Unlike previous trials, pexelizumab did not reduce the incidence of MI compared with placebo (12.6% vs 13.3%; P=.311).
The incidence of adverse events and serious adverse events was similar between groups.
Septicemia from infection was reduced significantly in pexelizumab-treated patients at 30 days (P=.05), 90 days (P=.03), and 180 days (P=.005).
The first PRIMO-CABG trial also showed a nonsignificant trend toward a reduction in death/MI with pexelizumab, but a subgroup analysis showed a significant reduction in this end point in patients deemed high-risk. PRIMO-CABG II was therefore conducted in patients with a higher surgical risk profile, Dr Smith said.
When the results of PRIMO-CABG II were pooled with other studies in patients undergoing CABG, involving a total of 7,959 patients, pexelizumab was associated with a 21% reduction in 30-day mortality (P=.043), he said.
In pooling all studies of pexelizumab (N=9,233), a significant 24% reduction in mortality (P=.0093) was observed in pexelizumab recipients relative to placebo.
OPTIMIZE-HF
Carvedilol at discharge associated with improved early survival in heart failure
Patients with heart failure who were prescribed carvedilol at the time of their discharge had better survival at 60 to 90 days than those not prescribed the drug at discharge, according to new data from the heart failure registry OPTIMIZE-HF (Organized Program to Initiate Life-saving Treatment in Hospitalized Patients with Heart Failure).
According to lead investigator Gregg C. Fonarow, MD, chairman of the OPTIMIZE-HF committee and director of the Ahmanson-University of California, Los Angeles, Cardiomyopathy Center: "Based on this analysis and earlier studies, carvedilol should be started when medically advisable at the time of hospital discharge in all eligible heart failure patients, since the difference in survival and hospitalizations emerges in just weeks among patients who receive carvedilol as opposed to those who don't have a beta blocker added to their treatment regimen."
Sixty- to 90-day postdischarge follow-up data were collected to measure the associations with clinical outcomes in eligible patients with left ventricular systolic dysfunction who received carvedilol prior to hospital discharge compared to those who were eligible but did not receive a beta blocker at discharge. Of the 2,720 patients with left ventricular systolic dysfunction identified, 2,373 were eligible to receive beta blocker therapy.
Carvedilol was prescribed at discharge to 1,146 patients, and 94.2% remained on it during follow-up. In the 361 eligible patients who were not discharged on any beta blocker, only 30.4% were started on beta blocker therapy postdischarge.
Carvedilol was associated with a 54% decreased risk of death (P=.006) and a 29% decreased risk of death or hospitalization (P=.02) without an early hazard for recurrent decompensation, Dr Fonarow said.
During another presentation, he said that beta blocker use for the treatment of heart failure is ideal for inclusion as a hospital performance measure.
In an analysis of a subset of 5,791 patients from the OPTIMIZE-HF database, none of the 5 existing ACC/American Heart Association heart failure performance measures were associated with a significant improvement in the risk of early mortality, and only the use of an ACE inhibitor or angiotensin receptor blocker was related to a reduction in the risk of death/rehospitalization at 60 to 90 days. The 5 measures are discharge instructions, evaluation of left ventricular systolic dysfunction, adult smoking cessation advice/counseling, and anticoagulant use in patients with atrial fibrillation.
In contrast, beta blocker use at hospital discharge, which is not currently a hospital performance measure for heart failure, was a strong predictor of mortality (P<.0001) and death/rehospitalization (P=.0004) at 60 to 90 days.
Incorporating beta blocker use as an inpatient heart failure performance measure offers the opportunity to significantly affect patient outcomes early in the postdischarge period, when patients are at highest risk, Dr Fonarow said.
FDA Approves Combination Therapy for Pulmonary Arterial Hypertension
March 25th 2024J&J’s Opsynvi is single-tablet combination of macitentan, an endothelin receptor antagonist, and tadalafil, a PDE5 inhibitor. It will be priced on parity with Opsumit, which is also a J&J product to treat patients with PAH.
FDA Issues Complete Response Letter for Onpattro in Heart Failure Indication
October 9th 2023Alnylam Pharmaceuticals will no longer pursue this indication of Onpattro and will instead on focus on a label expansion for Amvuttra, which is in phase 3 development to treat patients with cardiomyopathy of ATTR amyloidosis.
