- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
From the American Society of Hypertension 22nd Annual Scientific Meeting and Exposition: Nebivolol demonstrates long-term efficacy in treatment of hypertension; benefit extends to patients who are obese
Nebivolol is associated with long-term control of blood pressure and is as effective in obese patients as in nonobese patients with hypertension, according to the results of a 9-month extension study and a post-hoc analysis that were presented at the American Society of Hypertension 22nd Annual Scientific Meeting and Exposition.

Key Points
Nebivolol is associated with long-term control of blood pressure and is as effective in obese patients as in nonobese patients with hypertension, according to the results of a 9-month extension study and a post-hoc analysis that were presented at the American Society of Hypertension 22nd Annual Scientific Meeting and Exposition.
According to Vasilios Papademetriou, MD, professor of cardiology, Georgetown University, Washington, District of Columbia, first-generation beta-blockers have fallen out of favor for the initial treatment of hypertension because of their side effect profile and their lack of effect on outcomes in clinical trials of patients with hypertension. Although US and European guidelines for the treatment of hypertension still list beta-blockers as options for first-line treatment, the National Institute for Health and Clinical Excellence (NICE) and the National Collaborating Centre for Chronic Conditions, in conjunction with the British Hypertension Society no longer recommend beta-blockers as first-line therapy for hypertension.
Dr Papademetriou said nebivolol has unique mechanisms that set it apart from conventional beta-blockers. The agent improves endothelial function through an increase in nitric oxide bioactivity, he said.
The increase in cardiac output with nebivolol has been associated with less fatigue and less erectile dysfunction compared with nonvasodilating beta-blockers.
The data presented at the meeting were collected from 845 patients with mild-to-moderate hypertension who previously completed 1 of 3 randomized, placebo-controlled, 12-week, dose-ranging studies ("feeder" studies) of nebivolol 1.25 to 40 mg/d.
In the extension phase, 813 patients were treated with open-label nebivolol 5, 10, or 20 mg once daily. After 28 days, patients (n=206) who did not achieve an average sitting diastolic blood pressure (DBP) <90 mmHg received open-label diuretics or amlodipine as adjunctive therapy.
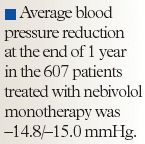
The average blood pressure reduction among the patients who received nebivolol plus a diuretic was –16.2/–12.0 mmHg, and 65.5% of the patients in this group were considered to be responders to treatment.
"These reductions in blood pressure indicate that nebivolol is as good as or better than other beta-blockers or other agents for that matter," Dr Papademetriou said.
Too few patients were treated with amlodipine to allow for meaningful comparisons.
The most common adverse events associated with nebivolol were fatigue (4.6% of patients), dizziness (3.0% of patients), and depression (0.9% of patients). A total of 31 (3.7%) patients (26 patients in the nebivolol monotherapy group, 4 patients in the nebivolol plus diuretic group, and 1 patient in the group receiving nebivolol plus amlodipine) withdrew from the study because of adverse events.
Nebivolol monotherapy was not associated with any clinically significant changes in glucose or lipids.
A post-hoc pooled analysis of three 12-week, randomized, double-blind, placebo-controlled trials demonstrated that blood pressure reductions with nebivolol were similar in obese (n=878) and nonobese (n=1,136) patients, said James R. Sowers, MD, director, Center for Diabetes and Cardiovascular Health, University of Missouri-Columbia.
By the end of the study, nonobese patients experienced a –7.7 to –11.5 mmHg reduction in trough sitting DBP versus a –4.9 mmHg reduction with placebo. Obese patients experienced a –6.7 to –9.5 mmHg reduction in trough sitting DBP versus a –4.0 mmHg reduction with placebo. Response rates were also comparable between the obese and nonobese patients.
FDA Approves Combination Therapy for Pulmonary Arterial Hypertension
March 25th 2024J&J’s Opsynvi is single-tablet combination of macitentan, an endothelin receptor antagonist, and tadalafil, a PDE5 inhibitor. It will be priced on parity with Opsumit, which is also a J&J product to treat patients with PAH.
FDA Issues Complete Response Letter for Onpattro in Heart Failure Indication
October 9th 2023Alnylam Pharmaceuticals will no longer pursue this indication of Onpattro and will instead on focus on a label expansion for Amvuttra, which is in phase 3 development to treat patients with cardiomyopathy of ATTR amyloidosis.
