- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Anidulafungin: An echinocandin antifungal for the treatment of Candida infection
Anidulafungin (Eraxis, Pfizer) is a new echinocandin approved for the treatment of Candida infection in adults. Like other echinocandins, anidulafungin acts on the fungal cell wall by inhibiting 1,3 beta-D glucan synthesis. Studies suggest that among the echinocandins, anidulafungin may have more potent in vitro activity against Candida spp and Aspergillus spp. Further, phase 2 and 3 clinical studies with anidulafungin have supported a high end of therapy success rates for invasive candidiasis, including esophageal candidiasis. Anidulafungin appears to be well tolerated, with headache, nausea, vomiting, phlebitis, neutropenia, and hypokalemia being the most commonly reported adverse effects. Importantly, as anidulafungin is chemically degraded, it has no clinically significant drug interactions and does not require any dose adjustment for renal or hepatic impairment.
Abstract
Anidulafungin (Eraxis, Pfizer) is a new echinocandin approved for the treatment of Candida infection in adults. Like other echinocandins, anidulafungin acts on the fungal cell wall by inhibiting 1,3 beta-D glucan synthesis. Studies suggest that among the echinocandins, anidulafungin may have more potent in vitro activity against Candida spp and Aspergillus spp. Further, phase 2 and 3 clinical studies with anidulafungin have supported a high end of therapy success rates for invasive candidiasis, including esophageal candidiasis. Anidulafungin appears to be well tolerated, with headache, nausea, vomiting, phlebitis, neutropenia, and hypokalemia being the most commonly reported adverse effects. Importantly, as anidulafungin is chemically degraded, it has no clinically significant drug interactions and does not require any dose adjustment for renal or hepatic impairment. (Formulary. 2006;41:387–403.)
In the United States, mortality associated with invasive fungal infections continues to increase, ranking as the 7th-leading cause of infectious disease-related death in 1997.1 Specific groups of patients have been found to be at an increased risk for invasive fungal infections.2,3 These patients include those with a recent transplant, receiving immunosuppressive medications or broad-spectrum antibiotics, having an intravenous catheter, as well as those with neutropenia, hematological cancers, or human immunodeficiency virus (HIV).2,3
Currently available therapies for the treatment of these common fungi include amphotericin B, azoles, and echinocandins. Recently, concern has been raised regarding resistance to both the azole and polyene (ie, amphotericin B) classes of antifungals.6 Additionally, although amphotericin B remains active against many Candida spp and Aspergillus spp, it is often not well tolerated due to its severe adverse events: infusion-related reactions, electrolyte disturbances, and nephrotoxicity.7 The lipid-based amphotericin B formulations have improved upon some of the tolerability concerns related to the traditional formulation; however, at higher doses, the toxicity profile continues to limit the agent's use.8
The azole class of antifungals is usually well tolerated. All of the agents in this class have activity against many Candida spp; however, itraconazole and voriconazole are the only currently approved azoles with coverage against Aspergillus spp.5 Unfortunately, because the azole class had a low toxicity profile, the agents have been used extensively, thus increasing the rates of resistance.6

On February 17, 2006, anidulafungin (Eraxis, Pfizer) received FDA approval for the treatment of Candida infections in adults.9 The specific approved indications for the agent are candidemia, Candida intra-abdominal abscesses, Candida peritonitis, and esophageal candidiasis.10 The approval of anidulafungin makes it the third echinocandin approved by FDA, joining caspofungin (Cancidas, Merck) (2001) and micafungin (Mycamine, Astellas Pharma) (2005).9 Table 1 features a comparison of the echinocandin antifungal agents.10–12
CHEMISTRY AND PHARMACOLOGY
Anidulafungin, formerly LY303366, has a molecular weight of 1140.30 daltons.13 The chemical formula is C58H73N7O17, with the structure of 1-[(4R,5R)-4,5-dihydroxy-N2-[[4"-(pentyloxy)[1,1':4',1"-terphenyl]-4-yl]carbonyl]-L-ornithine]echinocandin B.13
Similar to the other echinocandins, anidulafungin causes cell wall instability and death via noncompetitive inhibition of 1,3 beta-D glucan synthesis.6 Current research suggests that anidulafungin has activity against many common fungi, including Candida spp and Aspergillus spp.6 Moreover, some in vitro studies suggest that anidulafungin may have more potent activity than the other echinocandins.14–21
MICROBIOLOGY

Candida spp. One of the first in vitro studies evaluating anidulafungin activity was conducted from 1993 to 1995 at a teaching hospital in Turkey.21 This study determined the minimum inhibitory concentrations (MICs) of anidulafungin, fluconazole, and amphotericin B against yeasts obtained from patients with esophageal candidiasis.21 Of the 191 isolates, almost 70% were C albicans. MIC90 values against C albicans were 0.25, 1, and 1 mcg/mL for anidulafungin, fluconazole, and amphotericin B, respectively.


A further in vitro analysis was conducted from 2002 to 2003 at 64 medical centers in North America, Latin America, and Europe evaluating the activity of anidulafungin against 880 yeast and 68 mold isolates.18 The vast majority of isolates were C albicans, C parapsilosis, C tropicalis, and C glabrata. In addition, 38 isolates of A fumigatus were collected. In this study, anidulafungin demonstrated lower MIC values than fluconazole or itraconazole against all Candida species except C parapsilosis, C guilliermodii, and C lusitaniae.18 Data also suggested that anidulafungin may have excellent activity (MIC90, 0.03 mcg/mL) against A fumigatus.18
The Mycosis Study Group (MSG) of the National Institutes of Health compared the in vitro susceptibilities of 2,000 Candida isolates obtained from patients in the United States with candidemia.19 MIC values were determined for anidulafungin, caspofungin, micafungin, voriconazole, fluconazole, itraconazole, amphotericin B, and flucytosine, as well as for the investigational agent posaconazole. Similar to other studies, the most common Candida species were C albicans, C glabrata, C parapsilosis, and C tropicalis.19 Fluconazole had the highest MIC90 of any of the antifungal agents against C albicans (n=733) at 2 mcg/mL. This was the first study to include other echinocandin antifungals. The other echinocandins, caspofungin and micafungin, demonstrated low MIC values against most species, with only a few species having MIC90 of 2 mcg/mL. Specifically, both had MIC90 values of 2 mcg/mL against C parapsilosis and C lusitaniae, but only caspofungin also had an MIC90 of 2 mcg/mL against C krusei.19 MIC90 values for anidulafungin were between 0.03 and 0.25 mcg/mL, except against C parapsilosis at 2 mcg/mL.19 As shown in this study, the higher MIC90 against C parapsilosis appears to be an echinocandin class effect.
Additional studies have evaluated the in vitro activity of anidulafungin against fluconazole-resistant Candida spp. One study described 156 Candida isolates with a fluconazole MIC of 16 mcg/mL seen at a Spanish hospital over 5 years.14 At this institution, C albicans, C glabrata, and C krusei were the common species. All species also had an amphotericin B MIC90 of 1 mcg/mL. Additionally, anidulafungin MIC90 values were between 0.015 and 0.12 mcg/mL against all populations.14 The next study tested 315 fluconazole-resistant (MIC, 64 mcg/mL) Candida spp.15 Unlike previous studies, C krusei was most common. The anidulafungin MIC90 values were: C krusei (n=146, 0.12 mcg/mL), C glabrata (n=110, 0.12 mcg/mL), C albicans (n=41, 0.06 mcg/mL), and other Candida spp (n=18, 2 mcg/mL).15
Aspergillus spp. In early evaluations, anidulafungin demonstrated considerable activity against clinical isolates of Aspergillus and other filamentous fungi. Several studies have proposed that anidulafungin is considerably more active against Aspergillus spp than itraconazole and amphotericin B.20,24

Anidulafungin's in vitro activity was further studied against 68 Aspergillus spp collected over a 2-year period, at a Spanish hospital.25 The MIC values against Aspergillus spp for caspofungin, voriconazole, itraconazole, and amphotericin B were determined.25 Specific Aspergillus spp included: A fumigatus (n=28), A flavus (n=19), A niger (n=9), A glaucus (n=8), A terreus (n=2), and A flavipes (n=2). Anidulafungin exhibited MIC90 values of ≤0.03 mcg/mL against all Aspergillus spp.25 Voriconazole and itraconazole both exhibited MIC90 values of 0.12 mcg/mL against the isolates, while amphotericin B was the least active antifungal tested, with an MIC90 of 1 mcg/mL.25
Further studies included 2 small investigations into the in vitro activity of anidulafungin against opportunistic filamentous fungi, including major pathogens such as Aspergillus spp and Fusarium spp.20,26 The first study was comprised of 68 pathogenic, clinically obtained, opportunistic, filamentous fungi isolates collected in the United States over a 3-year period.26 Included in the 68 were 26 isolates of Aspergillus spp, 12 isolates of Fusarium spp, and other fungi (each 6 isolates). The activity of anidulafungin was determined and compared with micafungin and posaconazole. Similar to other studies, A fumigatus (n=13) was the most common species.26 Notably, although 2 of the 13 A fumigatus isolates were resistant to itraconazole (MIC >8 mcg/mL), they both had anidulafungin MIC values of 0.06 mcg/mL. MIC ranges for all Aspergillus spp isolates included: anidulafungin, <0.03 to 0.12 mcg/mL; micafungin, 0.5 to >16 mcg/mL; and posaconazole, <0.03 to 1.0 mcg/mL. Against Fusaruim spp, MIC ranges were ≥16 mcg/mL for the antifungals evaluated, except posaconazole (F oxysporum MIC range, 1–16 mcg/mL).26 The second study compared the activity of anidulafungin with micafungin, itraconazole, amphotericin B, and flucytosine against 51 filamentous fungi isolates. Like the previous study, Aspergillus spp (n= 24) and Fusarium spp (n=13) were the most common fungi isolated.20 The MIC ranges for anidulafungin against all Aspergillus spp were comparable to that of the previous study, ranging from 0.004 to 0.12 mcg/mL, with the higher MIC values associated with A fumigatus.20 The MIC values for anidulafungin against Fusarium isolates were all >2 mcg/mL. For other antifungals evaluated, the MIC ranges against Aspergillus spp were: micafungin, 0.03 to 0.5 mcg/mL; itraconazole, 0.25 to 1.0 mcg/mL; amphotericin B, 1 to 2 mcg/mL; and flucytosine, 32 to >128 mcg/mL.20 For Fusarium spp, MIC values were >8 mcg/mL for micafungin, 4 to >8 mcg/mL for itraconazole, 1 to 2 mcg/mL for amphotericin B, and >128 mcg/mL for flucytosine.20
SYNERGY
Although data regarding synergy are limited, there has been recent interest in the idea, especially due to the new site of action that the echinocandins provide. One study that included anidulafungin evaluated the agent in combination with itraconazole, voriconazole, or amphotericin B against Aspergillus and Fusarium spp.27 Thirty-three isolates (26 Aspergillus spp, 7 Fusarium spp) obtained from the University of Texas Medical School at Houston were evaluated using the checkerboard method to test for synergy.27 Fractional inhibitor concentration indices (FICI) were calculated and defined as follows: ≤0.5, synergy; 0.5 to 4.0, indifference; and >4.0, antagonism. Anidulafungin combined with itraconazole or voriconazole appeared to be synergistic against nearly all of A flavus (n=8) and A fumigatus (n=8) (1 A flavus isolate appeared indifferent with the voriconazole combination) isolates. Further, 62.5% of A fumigatus (n=8) isolates were synergistic when anidulafungin was combined with amphotericin B.27 However, no combination had synergism against either A niger (n=5) isolates or any Fusarium spp (n=7) isolates.27 Antagonism was only noted for the amphotericin B combination in 3 of 8 A flavus isolates and 2 of the 5 A terreus isolates.27
PHARMACOKINETICS



CLINICAL TRIALS
Three publications and several abstracts describe the clinical trials that evaluated the safety and efficacy of anidulafungin against Candida spp.16,28,42–44 The phase 2 and 3 adult clinical trials are summarized in Table 5.
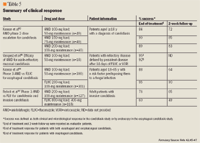
Phase 2. One hundred twenty-three adult patients with candidiasis were recruited between August 2001 and November 2002 at 24 sites in the United States.16,42 One hundred twenty of these patients were randomized and received at least 1 dose of anidulafungin at a load/maintenance daily dose of 100 mg/50 mg (n=40), 150 mg/75 mg (n=40), or 200 mg/100 mg (n=40) intravenously at a rate of 1 mg/min. There were no statistical differences between the dosage groups with regard to age (median 52–59 years), percent male (33%–53%), or weight (median 68.9– 76.1 kg).42 Patients in the higher dose groups had higher APACHE II scores than the low-dose group (APACHE II scores were 18%, 30%, and 25% in the 3 groups, respectively). Ninety-four percent of the patients had candidemia due to C albicans (53%), C glabrata (31%), C parapsilosis (9%), C tropicalis (9%), C krusei (4%), or another species (3%). Eighty-three patients were clinically evaluated at the end of treatment, but only 68 patients were seen 2 weeks after completion of therapy.42 Reasons for patients not completing the study included death, drug therapy of inadequate duration (not due to failure), additional antifungal agent (not due to failure), or no return for the final visit. The higher doses tended to have higher rates of global success than the lower 100-mg/50-mg dose.42Candida spp eradication rates were 74%, 85%, and 89% for the 50-mg, 75-mg, and 100-mg maintenance doses, respectively.16 Pathogen eradication did not appear to be well correlated with the MIC, as C parapsilosis with anidulafungin MIC values between 4 and 8 mcg/mL had response rates of ~85% (6 of 7).16 It is important to note, however, that eradication rates did parallel clinical and global success rates.16
Phase 3. Anidulafungin was evaluated in a phase 2/3 open-label, multi-center study for the treatment of fluconazole-resistant oropharyngeal and esophageal candidiasis. Patients had received fluconazole or voriconazole for at least 14 days and continued to have symptoms with positive evidence for yeast (smear or culture).45 Additionally, patients with esophageal candidiasis were required to have endoscopic evidence of disease. All patients were treated with anidulafungin at a 100-mg load followed by 50 mg daily for 2 to 3 weeks. A total of 18 patients were evaluated. Sixty-one percent had esophageal candidiasis. Twelve of the 18 patients had isolated a single species (11 C albicans and 1 C glabrata). Six patients had mixed infections; the majority of which (5 of 6) were C albicans with C glabrata (1 of 6). Fifty-two percent of C albicans and 40% of C glabrata were susceptible to fluconazole. The majority of patients had a successful clinical response, with only one patient being defined as a clinical failure. Further, only 1 of 11 patients treated for esophageal candidiasis did not have cure or improvement as defined by endoscopy.45
Six hundred one patients aged 18 to 65 years with esophageal candidiasis were randomized in a double-blind, double-dummy fashion to receive either intravenous anidulafungin (100-mg load/50-mg maintenance) or oral fluconazole (200-mg load/100-mg maintenance) daily.44,46 Subjects were recruited from the Republic of South Africa (n=453), Thailand (n=91), Argentina (n=51), and the United States (n=6). Diagnosis of esophageal candidiasis was based on clinical symptoms, endoscopy, and mycological findings. The predefined primary end point was end of therapy, per patient endoscopic response. Patients were evaluated at baseline, end of therapy, and 2 weeks after therapy cessation. Most baseline patient demographics and endoscopic severity were similar between groups. Although most patients in both groups had Acquired Immune Deficiency Syndrome (AIDS), more patients in the fluconazole group began antiretroviral therapy during the study (26 of those receiving anidulafungin vs 58 of those receiving fluconazole).46Candida species isolated included C albicans (91%), C glabrata (2%), C tropicalis (<1%), and undifferentiated or multiple Candida spp (7%). There were 249 and 250 patients evaluable at follow-up in the anidulafungin and fluconazole groups, respectively. The endoscopic success rates were similar between groups, with 97% and 99%, having a positive end of therapy endoscopic success. Sustained endoscopic success was less with anidulafungin versus fluconazole in the evaluable populations at 2 weeks (n=233 anidulafungin; 64.4% versus n=229; 89.5%).46 However, it is uncertain what role, if any, the administration of antiretroviral therapy has in the sustained response between groups.

Special populations. A pediatric study was conducted that included 25 children (aged 2–11 y) and 13 adolescents (aged 12–17 y) with neutropenia and at high risk for systemic fungal infections.28 The patients received 1 of 2 regimens. The daily dosage regimens were either low-dose (1.5-mg/kg/d load [max 100 mg]/0.75-mg/kg/d maintenance [max 50 mg] by IV infusion over 90 min/45 min, respectively) or high dose (3-mg/kg/d load [max 200 mg]/1.5-mg/kg/d maintenance [max 100 mg] by IV infusion over 180 min/90 min, respectively).28 No patients in either the high- or low-dose group experienced a systemic fungal infection during the study.
ADVERSE EVENTS
More than 400 adult patients received anidulafungin in phase 2 and 3 clinical trials. From these studies, adverse drug events were reported to occur in approximately 10% of patients.42,46 The most common adverse events reported in the phase 2 trial were: hypotension (13%), vomiting (13%), constipation (11%), nausea (11%), fever (11%), and hypokalemia (10%).42 The published phase 3 study reported only possible or probable treatment-related adverse events. The events that occurred in at least 1% of anidulafungin-treated patients were headache (1.3%), nausea (1%), phlebitis (1.3%), and neutropenia (1%).46 Additionally, 1 patient reported a flushing sensation but did not experience any other sign of infusion-related or allergic reaction. This patient was able to restart the infusion and continued the study without any further events.46
In addition to the common events, serious adverse events have also been reported. There were 5 serious adverse events reported in published phase 2 and phase 3 studies (n=423), including nonfatal neutropenia fever (n=1), seizures (n=2), maculopapular rash (n=1), and multisystem organ failure (n=1; the patient died from this, but causality could not be determined as the patient also had multiple comorbid conditions).42,46
In the pediatric phase 2 study, mild-to-moderate adverse events were reported by every patient.28 Although every patient reported at least 1 adverse event, further analysis by investigators suggests that only 4 patients had an event that was possibly/probably related to the drug.28 These events included feeling abnormal, facial erythema and rash (occurred once-did not reoccur with repeat administration), blood urea nitrogen elevation, fever, and hypotension.28
DRUG INTERACTIONS
To date, no clinically significant drug interactions have been described with anidulafungin. The potential for drug-drug interactions was evaluated with many medications, including voriconazole, lipid amphotericin B, cyclosporine. tacrolimus, and rifampin.29–32,41,48 Additionally, a large population (n=225) pharmacokinetic study demonstrated that the pharmacokinetics of anidulafungin were not affected by any cytochrome p450 substrates, inhibitors, or inducers.32
DOSING AND ADMINISTRATION
Adults. For all adult Candida infections except esophageal candidiasis, anidulafungin should be given as a 200-mg loading dose followed by 100 mg daily.10 The esophageal candidiasis dose is 100 mg on Day 1, followed by 50 mg each day thereafter.10
Special populations. Dosing has been studied in patients with all severities of renal insufficiency, including those who require dialysis.35,36 No dose adjustment is recommended for any degree of renal impairment or dialysis.10,35,36 Different severities of hepatic impairment were also studied, and no dose adjustment is recommended for any degree of hepatic impairment, even though small decreases in concentration were seen in patients with severe hepatic impairment.10,33,34,40 Although not yet FDA-approved in the pediatric population, preliminary data suggest that doses of 1.5 mg/kg/d and 0.75 mg/kg/d up to the adult doses will produce concentrations similar to the 100-mg/d and 50-mg/d adult doses, respectively.28
Anidulafungin is available in 50-mg vials with a 15-mL, 20% dehydrated alcohol in water diluent.10 Each 50-mg vial is to be diluted with the diluent then added to 85 mL of 5% dextrose in water (D5W) solution or normal saline.10 The final anidulafungin concentration of 0.5 mg/mL should be administered at a maximum rate of 1.1 mg/min.10
The package insert states that the medication should not be co-infused with electrolytes or any other medications.10 A recent study evaluated the compatibility of anidulafungin with 76 different additives. A Y-site was simulated using equal amounts of the each drug (anidulafungin was diluted to 0.5 mg/mL in D5W and 0.25 mg/mL in normal saline or D5W).49 Only 3 of the 76 medications suggested incompatibility, as defined by visible particulate, substantial haze, turbidity change, color change, or gas evolution. The 3 medications with incompatibility that should not be co-infused with anidulafungin at the Y-site are amphotericin B, ertapenem, and sodium bicarbonate.49
Dr St. Germain is a pediatric infectious disease fellow, at the University of Connecticut, Storrs, Conn, and at the Connecticut Children's Medical Center, Hartford, Conn. Dr Ellis is assistant clinical professor at the University of Connecticut School of Pharmacy and clinical pharmacy specialist in the Department of Pharmacy and Division of Pediatric Infectious Diseases, Connecticut Children's Medical Center. She can be reached at: jmellis@ccmckids.org
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, director of Drug Information Services at Hartford Hospital in Hartford, Conn, and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn; and by Craig I. Coleman, PharmD, assistant professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
EDITORS' NOTE: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
REFERENCES
1. McNeil MM, Nash SL, Hajjeh RA, et al. Trends in mortality due to invasive mycotic diseases in the United States, 1980-1997. Clin Infect Dis. 2001;33:641–647.
2. Clark TA, Hajjeh RA. Recent trends in the epidemiology of invasive mycoses. Curr Opin Infect Dis. 2002;15:569–574.
3. Fridkin SK. The changing face of fungal infections in health care settings. Clin Infect Dis. 2005;41:1455–1460.
4. Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systemic review of the literature. Clin Infect Dis. 2001;32:358–366.
5. Menichetti F. Emerging epidemiology of fungal infections [abstract S310]. Clin Microbiol Infect. 2004;10(suppl 3):57.
6. Canuto MM, Gutierrez Rodero F. Antifungal drug resistance to azoles and polyenes. Lancet Infect Dis. 2002;2:550–563.
7. Garbino J, Markham L, Matulionyte R, et al. Should we continue using amphotericin B deoxycholate for the treatment of fungal infections? Adverse events and clinical outcomes. Scand J Infect Dis. 2006;38:110–113.
8. Girois SB, Chapuis F, Decullier E and Revol BGP. Adverse effects of antifungal therapies in invasive fungal infections: review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2005;24: 119–130.
9. US Food and Drug Administration Center for Drug Evaluation and Research. A catalog of FDA approved drug productions. Available at: http:// http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist. Accessed July 14, 2006.
10. Eraxis [package insert]. New York, NY: Pfizer, Inc; 2006.
11. Mycamine [package insert]. Tokyo, Japan: Astellas Pharma Inc; 2005.
12. Cancidas [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2005.
13. United States Pharmacopeia. Anidulafungin. Drug Standards Pharmacopeial Forum 2000;26. Available at: http:// http://www.usp.org/USPNF/pf/2602/f01.html. Accessed July 14, 2006.
14. Cuenca-Estrella M, Mellado E, Diaz-Guerra TM, et al. Susceptibility of fluconazole-resistant clinical isolates of Candida spp. to echinocandin LY303366, itraconazole and amphotericin B. J Antimicrob Chemother. 2000;46:475–477.
15. Pfaller MA, Boyken L, Hollis RJ, et al. In vitro activities of anidulafungin against more than 2,500 clinical isolates of Candida spp., including 315 isolates resistant to fluconazole. J Clin Microbiol. 2005;43:5425–5427.
16. Pfaller MA, Diekema DJ, Boyken L, et al. Effectiveness of anidulafungin in eradicating Candida species in invasive candidiasis. Antimicrob Agents Chemother. 2005;49:4795–4797.
17. Odabasi Z, Paetznick VL, Rodriguez JR, et al. In vitro activity of anidulafungin against selected clinically important mold isolates. Antimicrob Agents Chemother. 2004;48:1912–1915.
18. Messer SA, Kirby JT, Sader HS, et al. Initial results from a longitudinal international surveillance programme for anidulafungin (2003). J Antimicrob Chemother 2004;54:1051–1056.
19. Ostrosky-Zeichner L, Rex JH, Pappas P, et al. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob Agents Chemother. 2003:3149-3154.
20. Pfaller MA, Marco F, Messer SA and Jones RN. In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792) against clinical isolates of Aspergillus, Fusiarium, Rhizopus, and other filamentous fungi. Diagn Microbiol Infect Dis. 1998;30:251-255.
21. Uzun O, Kocagoz S, Cetinkaya Y, et al. In vitro activity of a new echinocandin, LY303366, compared with those of amphotericin B and fluconazole against clinical yeast isolates. Antimicrob Agents Chemother. 1997;41:1156–1157.
22. Zhanel GG, Karlowsky JA, Zelenitsky SA, et al. Susceptibilities of Candida species isolated from the lower gastrointestinal tracts of high-risk patients to new semisynthetic echinocandin LY303366 and other antifungal agents. Antimicrob Agents Chemother. 1998;42:2446–2448.
23. Marco F, Danes C, Almela M, et al. Trends in frequency and in vitro susceptibilities to antifungal, including voriconazole and anidulafungin, of Candida bloodstream isolates. Results from a six-year study (1996-2001). Diagn Microbiol Infect Dis. 2003;46:259-264.
24. Oakley KL, Moore CB, Denning DW. In vitro activity of the echinocandin antifungal agent LY303,366 in comparison with itraconazole and amphotericin B against Aspergillus spp. Antimicrob Agents Chemother. 1998;42:2726–2730.
25. Serrano MC, Valverde-Conde A, Chavez M, et al. In vitro activity of voriconazole, itraconazole, caspofungin, anidulafungin (VER002, LY303366) and amphotericin B against aspergiullus spp. Diagn Microbiol Infect Dis. 2003;45:131–135.
26. Espinel-Ingroff A. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol. 1998;36:2950–2956.
27. Philip A, Odabasi Z, Rodriguez J, et al. In vitro synergy testing of anidulafungin with itraconazole, voriconazole, and amphotericin B against Aspergillus spp. and Fusarium spp. Antimicrob Agents Chemother. 2005;49:3572–3574.
28. Benjamin DK, Jr., Driscoll T, Seibel NL, et al. Safety and pharmacokinetics of intravenous anidulafungin in children with neutropenia at high risk for invasive fungal infections. Antimicrob Agents Chemother. 2006;50:632–638.
29. Dowell J, Schranz J, Stogniew M, et al. Assessment of the pharmacokinetics of anidulafungin in patients with invasive aspergillosis receiving concomitant liposomal amphotericin [abstract P1036]. Clin Microbiol Infect. 2004;10:278.
30. Dowell JA, Schranz J, Baruch A, Foster G. Safety and pharmacokinetics of coadministered voriconazole and anidulafungin. J Clin Pharmacol. 2005;45:1373–1382.
31. Dowell JA, Stogniew M, Krause D, et al. Assessment of the safety and pharmacokinetics of anidulafungin when administered with cyclosporine. J Clin Pharmacol. 2005;45:227–233.
32. Stogniew M, Dowell J, Krause D, Henkel TJ. Population pharmacokinetics confirms absence of anidulafungin drug-drug interactions [abstract P1035]. Clin Microbiol Infect. 2004;10(suppl 3):278.
33. Thye D, Kilfoil T, Kilfoil G, Henkel T. Anidulafungin: Pharmacokinetics (PK) in subjects with severe hepatic impairment (HI). Presented at: 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; San Diego, Calif. September 27–30, 2002. Abstract A-1392.
34. Thye D, Kilfoil T, White RJ and Lasseter K. Anidulafungin: Pharmacokinetics in subjects with mild and moderate hepatic impairment. Presented at: 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, Ill. December 16–19, 2001. Abstract A-34.
35. Thye D, Marbury T, Kilfoil T, et al. Anidulafungin: Pharmacokinetics in subjects receiving hemodialysis. Presented at: 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; San Diego, Calif. September 27–29, 2002. Abstract A-1390.
36. Thye D, Marbury T, Kilfoil T, et al. Anidulafungin: Pharmacokinetics in subjects with renal impairment. Presented at: 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy; San Diego, Calif. September 27–30, 2002. Abstract A-1391.
37. Dowell JA, Knebel W, Ludden T, et al. Population pharmacokinetic analysis of anidulafungin, an echinocandin antifungal. J Clin Pharmacol. 2004;44:590–598.
38. Dowell J, Schranz J, Wilson J, et al. Anidulafungin (ANID) pharmacokinetics are not affected by concomitant voriconazole (VORI) [abstract P1034]. Clin Microbiol Infect. 2004;10(suppl 3):277–278.
39. Dowell J, Pu F, Lee J, et al. A clinical mass balance study of anidulafungin (ANID) showing complete fecal elimination. Presented at: 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, Ill. September 14–17, 2003. Abstract A-1576.
40. Krause D, Schranz J and Birmingham W. Lack of hepatic effect of anidulafungin [abstract P1789]. Clin Microbiol Infect. 2004;10(suppl 3):509.
41. White RJ, Thye D. Anidulafungin does not affect the metabolism of cyclosporin by human hepatic microsomes. Presented at: 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, Ill. December 16-19, 2001. Abstract A-35.
42. Krause DS, Reinhardt J, Vazquez JA, et al. Phase 2, randomized, dose-ranging study evaluating the safety and efficacy of anidulafungin in invasive candidiasis and candidemia. Antimicrob Agents Chemother. 2004;48:2021–2024.
43. Thye D, Shepard B, White RJ, et al. Anidulafungin: A phase 1 study to identify the maximum tolerated dose in healthy volunteers. Presented at: 41st Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, Ill. December 16–19, 2001. Abstract A-36.
44. Krause D, Henkel T, Goldstein B and Walsh T. Anidulafungin (ANID) vs. fluconazole (FLU) in esophageal candidiasis (EC): a phase 3, randomized, double-blind, multicenter trial. Presented at: 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, Ill. September 14–17, 2003. Abstract M-1760.
45. Vazquez J, Schranz J, Krause D, et al. Efficacy of anidulafungin (ANID) in patients (pts) with azole-refractory mucosal candidiasis (ARMC). Presented at: 44th Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington, DC. October 30–November 2, 2004. Abstract M-1038.
46. Krause DS, Simjee AE, van Rensburg C, et al. A randomized, double-blind trial of anidulafungin versus fluconazole for the treatment of esophageal candidiasis. Clin Infect Dis. 2004;39:770–775.
47. Reboli A, Rotstein C, Pappas P, et al. Anidulafungin vs. fluconazole for treatment of candidemia and invasive candidiasis (C/IC). Presented at: 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington, DC. December 16–19, 2005. Abstract M-718.
48. Dowell J, Schranz J, Buckwalter M, et al. Tacrolimus pharmacokinetics are not affected by concomitant anidulafungin [abstract P1113]. Clin Microbiol Infect. 2005;11(suppl 2):349.
49. Trissel L, Ogundele A. Compatibility of anidulafungin with other drugs during simulated Y-site administration. Am J Health-Syst Pharm. 2005;62:834–837.
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
