- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Anticoagulation prophylaxis practice patterns in patients having total hip, total knee replacement in a US health plan
This study aims to illuminate anticoagulation prophylaxis practice patterns in the United States after total joint replacement surgery.

Key Points
Abstract
This study aims to illuminate anticoagulation prophylaxis practice patterns in the United States after total joint replacement surgery, with specific attention to duration of overlapping therapy for patients who are "bridged" from low-molecular-weight heparin (LMWH) to warfarin. A large hospital drug database and linked outpatient files were retrospectively analyzed and compared for demographic, clinical, and provider characteristics. Of 2,280 enrollees, 1,770 met eligibility criteria, 1,551 received anticoagulant prophylaxis, 692 received only LMWH, and 595 received only warfarin. Of patients who were bridged from one agent to the other, 94% had less than 5 days of overlap. Each day of overlapping prophylaxis was associated with a 0.857 times decreased risk-adjusted incidence rate of thromboembolic events. Rates were 0.957 times lower for major bleeding and 0.895 times lower for minor bleeding for each day of overlap. In this analysis, longer duration of overlapping therapy is associated with decreased adverse event rates of both venous thromboembolism and bleeding. (Formulary. 2011;46:486–500.)
After total hip or total knee replacement (THR/TKR) surgery, patients are at high risk for venous thromboembolism (VTE) events, and this high risk can persist for up to 3 months after surgery.1,2 VTE events occur in up to 40% of patients who do not receive preventive care following major orthopedic surgery, yet studies show that orthopedic patients receive inadequate prophylactic therapy.3-5 More-recent studies show much lower rates of prophylactic therapy among outpatient orthopedic surgery patients compared with rates among inpatients.6
Established guidelines recommend thromboprophylaxis regimens for individuals at risk of VTE, which comprises deep vein thrombosis (DVT) and pulmonary embolism (PE).1,7-9 Chemoprophylaxis regimens usually consist of 4 to 5 days of overlap with both a low-molecular-weight heparin (LMWH) and warfarin. Warfarin is appropriate for out-of-hospital use because it is administered orally; however, it has a narrow therapeutic window as well as numerous food and drug interactions, and patients must be monitored to ensure that they are within the international normalized ratio (INR) of 2.0 to 3.0, as recommended by the American College of Chest Physicians (ACCP).1 LMWH can be safely given in the outpatient setting with laboratory testing of partial prothrombin time and a reduced requirement for direct physician observation, but unlike warfarin, it is administered subcutaneously, and it has not been extensively used as long-term therapy. Evidence-based guidelines recommend that anticoagulation be continued for at least 10 days following THR or TKR surgery, and up to 35 days for THR patients.1,2,10
The Global Orthopaedic Registry (GLORY) reported on the incidence of VTE and patterns of prophylaxis based on data from 6,639 THR and 8,326 TKR patients.10 VTE occurred after the median time to discharge in 75% of the THR and 57% of the TKR patients who developed it. Yet, of the patients who did receive appropriate in-hospital prophylaxis, 26% of THR and 27% of TKR patients were no longer receiving it 7 days after surgery.
In another recent study by Tapson and colleagues, of 3,778 patients at 38 US hospitals who were admitted for a variety of reasons, including THR or TKR surgery, a large percentage did not receive adequate primary preventive therapy for thromboembolic disease, and among patients who experienced VTE, discharge from the hospital with appropriate anticoagulant bridging therapy was rarely practiced.5 Furthermore, in 49.4% of patients with DVT, PE, or both, unfractionated heparin or LMWH was discontinued too early, before an INR of =2.0 for 2 consecutive days, as recommended in the ACCP guidelines, was attained. Bridging therapy is crucial in this setting to protect patients from risk of VTE.11–13
Using a hospital drug database linked with outpatient claims, we examined real-world anticoagulation use and the effect of bridging therapy on clinical outcomes. A unique feature of this data set is that it allowed us to follow patients from in-hospital to outpatient therapy.
METHODS AND DATA SOURCES
Data sources and study population. This study used a subset of the MarketScan Hospital Drug Database and its linked outpatient files from the MarketScan Commercial and Medicare Supplemental database from Thomson Reuters. This is a proprietary database containing the largest collection of employer-based patient data in the United States. The following patient-level identifiers were used to match the claims and inpatient databases: admission date, discharge date, age, sex, and principal diagnosis. Admissions that were not uniquely identified by the set of key variables were excluded. If the values of all key variables were duplicated across multiple admissions within a hospital, it was not possible to establish a unique match.14
The linkage was conducted for 172 hospitals identifiable in both the hospital and claims databases. Data were collected between January 1, 2005, and December 31, 2007. For 2007, data for nearly 22,189 patient-level hospital records were successfully linked to longitudinal claims histories. Hospital data comprised submitted claims (UB02 format, which itemizes data such as reason for and date of admission, condition codes, revenue codes, payer information, ICD-9-CM diagnosis codes, and procedure codes, etc.) linked to detailed service-level hospital bills for the same admissions. For each contributing hospital, the hospital database contained the full census annual admissions. Claims data were fully adjudicated, and paid claims from covered medical and pharmacy services were from geographically dispersed private and public health plans.15
Inclusion and exclusion criteria. The study population included commercial and Medicare patients with continuous enrollment in their health plan for at least 180 days prior to and 90 days following the index date (the date of surgery). For THR surgery, patients had to be admitted with International Classification of Disease 9th Revision, Clinical Modification (ICD-9-CM) codes: 81.42–81.47, 81.54–81.55, or, for TKR surgery, ICD-9-CM codes: 81.40, 81.51–81.53. Patients were excluded from the analysis if they were aged 18 or younger at the index date.
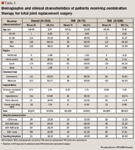
Demographic characteristics (age, gender, geographic location) were available in the enrollment data of the claims database. Using the Deyo adaptation of the Charlson Comorbidity Index,16-18 baseline comorbidities were measured for each patient. The Charlson Comorbidity Index assigns a weight ranging from 1 to 6 according to disease severity for 19 conditions. Its contents and weighting scheme are based on Cox proportional hazards modeling. The weights for each condition are summed, and a score is assigned to each patient.
Using appropriate ICD-9-CM codes, we also identified patients with hypertension, renal disease, and cancer, excluding myelodysplastic syndrome, for which anticoagulants are contraindicated, during the baseline period. Hospital characteristics (number of beds and teaching status) were available in the hospital drug database.

Patients were divided based on their treatment pattern: (a) LMWH-only users, (b) warfarin-only users, and (c) combination therapy (both LMWH and warfarin) users. Combination therapy was further divided as: (1) bridged (overlap of at least 2 days in hospital just prior to discharge or overlap of 2 days in outpatient setting), and (2) switched (<2 days of overlap in inpatient or outpatient settings).
Baseline characteristics were compared among the patient cohorts, and descriptive statistics were calculated as means (±SD) and percentages. As traditional regression assumes a symmetric distribution and constant variance, we used Poisson regression, which fits models of the number of occurrences (counts) of an event, to estimate the risk-adjusted event rates within 90 days of discharge. We also estimated the incidence rate, ie, the rate at which events occur. The unit of measurement was overlap days. At each overlap day, the investigators calculated event rate, demographics (age, gender, insurance type), and clinical factors, such as comorbid conditions. Event rates were compared using incidence-rate ratio. Incidence-rate-ratio analyses provided information about incidence rate of the events for each day of overlap. Since the sample size was small, the investigators used the bootstrapping method, which chooses a pair of observations from the sample with replacement and computes estimates for each sample, as described by Baser et al, to estimate the standard errors.19 We repeated this replication 250 times, although 5 to 200 replications are generally considered adequate for estimates of standard error.20 Statistical analyses were performed using SAS v9.2 and STATA v10.
RESULTS
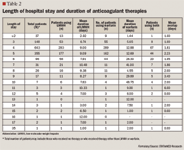
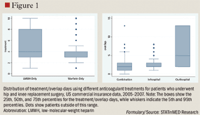
The linked database contained 2,280 enrollees, of whom 1,770 met the eligibility criteria: 491 patients underwent THR and 1,279 underwent TKR surgery. Of these eligible patients, 1,551 (87.6%) received anticoagulants: 595 (34%) received only warfarin, 692 (39%) received only LMWH, and 264 (15%) received a combination of anticoagulants. Of the 264 patients who received combination anticoagulants, 75 were in the THR surgery group and 189 were in the TKR surgery group.
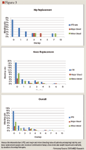

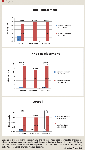
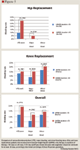
DISCUSSION
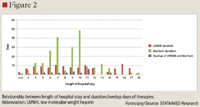
Using a hospital drug database linked with outpatient claims, we evaluated the overlap of anticoagulant care following THR and TKR surgeries and the effects of bridging or not bridging on real-world outcomes. In an earlier study of the same patient population and database, we found that only a small minority of patients who underwent THR and TKR surgery received appropriate bridging therapy as outpatients, although both VTE events and bleeding events were lower when bridging prophylaxis was used.20 As in that study, the present study shows that practice patterns vary widely and often are not consistent with major guidelines. Longer overlap of anticoagulation therapy decreased adverse event rates (incidence ratio rates ranged from 0.857–0.957).
Results from this current study show that in a real-world setting most patients receive inadequate bridging therapy. Overall duration of therapy is inadequate as well: according to published guidelines, anticoagulation should be continued for at least 10 days and up to 35 days postsurgery for THR patients.21 In our sample, however, 106 THR patients (52%) receiving LMWH only and 150 patients (94%) receiving warfarin only had a total duration of therapy of <10 days.
In 2008, the Centers for Medicare & Medicaid Services (CMS) took a step toward improving patient safety by establishing the category of "never events." These are hospital-acquired conditions that are considered preventable and should never have occurred. The CMS will no longer reimburse hospitals for VTE with THR and TKR surgeries. Therefore, there has been an increased awareness of never events in inpatient VTE following total joint replacement surgery, but the majority of VTE events occur after hospital discharge. 22
LIMITATIONS
There are limitations to our study. First, our sample size is relatively small, reducing the power to detect more subtle differences in event rates among patients with different regimens. As a result, instead of evaluating outcomes closely related to hospital discharge, we had to use overall complication rates at 90 days as a primary end point.
The next set of limitations is typical of any retrospective claims database.16 While claims data are extremely valuable for treatment patterns, healthcare resource utilization, and costs, these data are collected for the purpose of payment rather than research. Presence of a diagnosis code on a medical claim is not necessarily proof of the presence of disease, as diagnoses may be incorrectly coded or included as rule-out criteria rather than actual disease. However, we applied detailed quality checks to the data set before starting the analysis, in order to mitigate these problems. Medications filled over-the-counter (eg, aspirin) or provided as samples by physicians were not measurable in the claims data. However, our analysis is descriptive and based on comparing combination therapy with monotherapy; therefore, we do not believe this lack of information on over-the-counter medicine significantly changed our results. Finally, data on the timing of VTE events and bleeding relative to bridging or no bridging were not available.
This study suggests that in a large proportion of patients undergoing THR or TKR surgery in the United States, anticoagulation therapy in general as well as anticoagulant bridging therapy are discontinued too early. Extending the duration of anticoagulation would likely significantly decrease complication rates without increasing healthcare costs. Physicians' attitudes, fears about increased risk of bleeding, and adherence problems resulting from complexities in the use of current therapies might contribute to this lack of adherence.23 New oral anticoagulants are emerging that may simplify effective anticoagulation therapy for patients following THR and TKR. These include the direct factor Xa inhibitors rivaroxaban and apixaban and the direct thrombin inhibitor dabigatran.
Recently published clinical trial data in both THR and TKR showed that the oral, direct factor Xa inhibitor rivaroxaban 10 mg significantly reduced the risk of VTE versus 40- or 30-mg enoxaparin without significant increase in major bleeding.24–27 Findings from the rivaroxaban clinical program also support the use of extended-duration prophylaxis as superior to short-term prophylaxis in the management of patients undergoing total hip arthroplasty.25 Another direct factor Xa inhibitor in development, apixaban, was superior to enoxaparin for preventing VTE after TKA in a phase 3 study, ADVANCE-2,but it failed to meet criteria for noninferiority versus enoxaparin in patients after TKA in the ADVANCE-1 trial. 28,29 The direct thrombin inhibitor dabigatran reduced VTE and all-cause mortality in THR and TKR patients, respectively, in the double-blind RE-NOVATE and RE-MODEL trials, but failed to show similar efficacy to the twice-daily North American dose of enoxaparin in patients undergoing TKR in the RE-MOBILIZE trial, although it was effective versus a once-daily dose.30–32 Since the new oral anticoagulants do not require routine anticoagulation monitoring or INR testing, they have the potential to reduce the need for bridging and the complexities associated with it post-hospital discharge.
CONCLUSION
Real-world practices in bridging patients from LMWHs to warfarin therapy vary widely. Few patients are bridged, and for those who are, overlapping therapy may be for too short a time. A longer duration of overlapping therapy appears to result in a lower incidence of post-discharge VTE.
Dr Baser is adjunct professor of internal medicine, the University of Michigan, and president, STATinMED Research, Ann Arbor, Mich; Dr Wang is a director of analytic research, STATinMED Research, Dallas; Dr Supina is associate director, Johnson & Johnson Pharmaceutical Services, and Dr Sengupta is director, Johnson & Johnson Pharmaceutical Services, Raritan, NJ.
The authors would like to acknowledge Ruth Sussman, PhD, for editorial assistance in the preparation of this manuscript with funding from Ortho-McNeil Janssen Scientific Affairs.
Dr Baser and Dr Wang report that they are employees of STATinMED Research and STATinMED Research is a consultant for Johnson & Johnson.
REFERENCES
1. Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-BasedClinical Practice Guidelines (8th Edition). Chest. 2008;133:381S–453S.
2. Kearon C. Duration of venous thromboembolism prophylaxis after surgery. Chest. 2003;124:386S–392S.
3. Bartley MK. Keep venous thromboembolism at bay. Nursing. 2006;36:36–41.
4. Stratton MA, Anderson FA, Bussey HI, et al. Prevention of venous thromboembolism. Adherence to the 1995 American College of Chest Physicians Consensus Guidelines for Surgical Patients. Arch Intern Med. 2000;160:334–340.
5. Tapson VF, Hyers TM, Waldo AL, et al, for the NABOR (National Anticoagulation Benchmark and Outcomes Report) Steering Committee. Antithrombotic therapy practices in US hospitals in an era of practice guidelines. Arch Intern Med. 2005;165:1458–1464.
6. Amin AN, Lin J, Ryan A. Need to improve thromboprophylaxis across the continuum of care for surgical patients. Adv Ther. 2010;27:81–93.
7. Johanson NA, Lachiewicz PF, Lieberman JR, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. J Bone Joint Surg Am. 2009;91:1756–1757.
8. National Institute for Health and Clinical Excellence. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. NICE clinical guideline 92. http://www.nice.org.uk/guidance/index.jsp?action=byID&o=12695. Accessed May 24, 2011.
9. Parvizi J, Azzam K, Rothman RH. Deep venous thrombosis prophylaxis for total joint arthroplasty: American Academy of Orthopaedic Surgeons guidelines. J Arthroplasty. 2008;23:2–5.
10. Warwick DF, Friedman RJ, Agnelli GF, Gil-Garay E, Johnson K, FitzGerald G, Turibio FM. Insufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared with the time course of thromboembolic events: findings from the Global Orthopaedic Registry. J Bone Joint Surg [Br]. 2007;89:799–807.
11. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:160S–198S.
12. Hirsh J, Guyatt G, Alberts GW, et al. Executive Summary. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133:71S-105S.
13. Hyers TM, Agnelli G, Hull RD, Morris TA, Tapson V. Antithrombotic therapy for venous thromboembolic disease. Chest. 2001;119:176S–193S.
14. Huse D, Curkendall S. Linking person-level inpatient data to longitudinal records. International Society for Pharmacoeconomics and Outcomes Research 12th Annual Congress, 2009, A391.
15. Amin AN, Lin J, Lenhart G, Schulman KL. Clinical and economic outcomes in patients at risk of venous thromboembolism receiving appropriate enoxaparin or unfractionated heparin prophylaxis. Thromb Haemost. 2009;102:321–326.
16. Baser O, Palmer L, Stephenson J. The estimation power of alternative comorbidity indices. Value Health. 2008;11:946–955.
17. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251.
18. D'Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol. 1996;49:1429–1433.
19. Baser O, Crown WH, Pollicino C. Guidelines for selecting among different types of bootstraps. Curr Med Res Opin. 2006;22:799–808.
20. Baser O, Supina D, Sengupta N, Wang L. Analysis of anticoagulation bridging therapy for patients undergoing total hip or total knee replacement in a U.S. health plan: real world observations and implications. American Health & Drug Benefits. 2011; In Press.
21. Geerts WH, Pineo GF, Heit JA, Berqvist D, Lassen MR, Colwell CW, Ray JG. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338S–400S.
22. Medicare and Medicaid move aggressively to encourage greater patient safety in hospitals and reduce never events. Centers for Medicare & Medicaid Services Website, July 31, 2008. Available at: http://www.cms.hhs.gov/apps/media/press/release.asp?Counter=3219&intNumPerPage=10&checkDate=&checkKey=&srchType=1&numDays=3500&srchOpt=0&srchData=&keywordType=All&chkNewsType=1%2C+2%2C+3%2C+4%2C+5&intPage=&showAll=&pYear=&year=&desc=false&cboOrder=date.
23. Caprini JA, Tapson VF, Hyers TM, et al, for the NABOR Steering Committee. Treatment of venous thromboembolism: adherence to guidelines and impact of physician knowledge, attitudes, and beliefs. J Vasc Surg. 2005;42:726–733.
24. Eriksson BI, Borris LC, Friedman RJ, et al, for the RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775.
25. Kakkar AK, Brenner B, Dahl OE, et al, for the RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008;372:31–39.
26. Lassen MR, Ageno W, Borris LC, et al, for the RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776–2786.
27. Turpie AG, Lassen MR, Davidson BL, et al; RECORD4 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet. 2009;373:1673–1680.
28. Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Harnick P, and the ADVANCE-2 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375:807–815..
29. Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604.
30. Eriksson BI, Dahl OE, Rosencher N, et al, for the RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–956..
31. Eriksson BI, Dahl OE, Rosencher N, et al, for the RE-MODEL Study Group. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost. 2007;5:2178–2185.
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
