- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Apixaban: An orally active factor Xa inhibitor to prevent strokes and systemic embolism in patients with nonvalvular atrial fibrillation
Apixiban (Eliquis) is an orally active factor Xa inhibitor. Its manufacturers are seeking FDA approval to market apixaban to reduce risk of stroke and systemic embolism in patients with nonvalvular AF (NVAF).
Abstract
Atrial fibrillation (AF) is the most common clinically significant cardiac arrhythmia in the United States, and it increases the risk of stroke by about 5-fold. Stroke can result in substantial morbidity and mortality. It decreases patients' quality of life and results in increased healthcare costs. Warfarin is effective for stroke prevention in patients with AF, but it has several drawbacks, including pharmacokinetic and pharmacogenomic interactions and the necessity of frequent laboratory monitoring. Consequently, there is a need for new anticoagulant agents that are effective, safe, and convenient to use. Apixaban (Eliquis) is an orally active factor Xa inhibitor. Its manufacturers are seeking FDA approval to market apixaban to reduce risk of stroke and systemic embolism in patients with nonvalvular AF (NVAF). In the AVERROES trial, apixaban reduced the rate of stroke or systemic embolism compared with aspirin in patients with NVAF who were unwilling to take warfarin or deemed unsuitable for it, and apixaban was not associated with an increased risk of major bleeding. In the ARISTOTLE trial, patients with NVAF and at least 1 other risk factor for stroke, who took apixaban 5 mg twice daily, had fewer primary efficacy events (stroke or systemic embolism), a lower rate of major bleeding (including intracranial hemorrhage), and decreased all-cause mortality compared with those receiving adjusted-dose warfarin. Apixaban is a substrate for CYP3A4 and P-glycoprotein, so the potential for drug-drug interactions exists. (Formulary. 2012;47:180–183.)
It is estimated that 1 in 4 Americans older than 40 will develop atrial fibrillation (AF).1 Prevalence estimates suggest that about 3 million people in the United States have AF, and by 2050, the projected number of Americans with AF is expected to exceed 12 million.1 AF imposes a significant financial burden on the healthcare system through substantially increased utilization rates for inpatient, emergency, and other medical services. Total costs of AF treatment in the United States have been estimated at $6.65 billion per year ($2.93 billion in direct costs, $1.95 billion in indirect costs, and $1.53 billion in outpatient costs; with only $235 million spent on medications).2
Apixaban (Eliquis), which is being developed jointly by Bristol-Myers Squibb and Pfizer, is an orally active direct factor Xa inhibitor. Its manufacturers are seeking FDA marketing approval for apixaban to reduce risk of stroke and systemic embolism in patients with AF. Apixaban is a promising new oral anticoagulant that may become an attractive alternative to warfarin and may have a significant impact on the current approach to managing stroke prevention in patients with AF. The FDA plans to make a decision regarding the new drug application by June 28, 2012.
PHARMACOLOGY AND PHARMACOKINETICS
Apixaban is a selective and reversible inhibitor of factor Xa, a key coagulation factor located at the junction of the extrinsic and intrinsic pathways of the coagulation cascade. By blocking factor Xa, apixaban decreases the generation of thrombin.6
Apixaban has a bioavailability of 66%, which is not affected by taking the drug with food.7,8 Apixaban has a rapid onset of action, with time to reach peak concentration (Tmax) of 1-3 hours, a low volume of distribution, clearance of 5 L/hour, and half-life between 8 and 15 hours.7, 9-11 A large majority of apixaban (87%) is protein bound.12 About 25% of apixaban's elimination occurs through the kidneys, with the remainder (about 55%) excreted via the fecal route.10 The presence of multiple elimination pathways suggests that patients with hepatic or renal impairment may be treated with apixaban.10 Apixaban is predominantly metabolized by CYP3A4/5 to several metabolites, the most prominent of which is O-demethyl apixaban sulphate.9,12 In addition, CYP1A2 and CYP2J2 play a minor role in apixaban's metabolism, with CYP2C8, 2C19, 2C9, and 2D6 having no involvement.10,13 Apixaban does not inhibit CYP1A2, 2C8, 2C9, 2C19, 2D6, or 3A4.11,14
CLINICAL TRIALS
Use of apixaban for stroke prevention in patients with NVAF has been evaluated in 2 multicenter randomized controlled trials.
The first trial was the double-blind Apixaban Versus Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial.15 Investigators randomly assigned 5,599 patients with NVAF at an increased risk of stroke (average CHADS2 score, 2.1), and for whom warfarin therapy was deemed unsuitable/undesirable, to receive apixaban 5 mg twice daily or aspirin 81 mg to 324 mg once daily.The reasons for which warfarin was determined to be unsuitable or undesirable in the patients enrolled included the following: international normalized ratio (INR) measurements could not be obtained or were unlikely to be obtained at the requested intervals (43%); only moderate risk of stroke (21%); and patients did not want to take warfarin (37%).
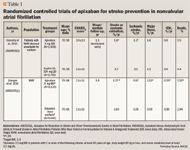
The double-blind, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial compared the efficacy and safety of apixaban 5 mg twice daily versus adjusted-dose warfarin with a target INR of 2.0 to 3.0 in 18,201 patients with NVAF and at least 1 additional risk factor for stroke (average CHADS2 score, 2.1).16 Patients meeting 2 or more of the following criteria received apixaban 2.5 mg instead of 5 mg: age at least 80 years, body weight 60 kg or less, and serum creatinine level at least 1.5 mg/dL).
After a median of 1.8 years of patient follow-up, the rate of the primary outcome (stroke or systemic embolism) was decreased with apixaban use (HR with apixaban, 0.79; 95% CI, 0.66–0.95; P<.001 for noninferiority; P=.01 for superiority). Furthermore, the rate of major bleeding was reduced with apixaban compared with warfarin (HR, 0.69; 95% CI, 0.60–0.80), as was the rate of ICH (HR, 0.42; 95% CI, 0.30–0.58). As a result of its superior efficacy and safety, patients randomized to receive apixaban had lower mortality rates (HR, 0.89; 95% CI, 0.80–0.99; P=.047).16
ADVERSE EVENTS
As mentioned previously, bleeding is a common adverse effect associated with apixaban use. The rates of major bleeding in the clinical trials, however, were less than those observed with warfarin and similar to those associated with aspirin. Compared with aspirin, apixaban was associated with more episodes of both minor bleeding (HR, 1.24; 95% CI, 1.00–1.53) and nonmajor but clinically relevant bleeding (HR, 1.15; 95% CI, 0.86–1.54; P=.35). Compared with warfarin, apixaban was associated with a lower rate of bleeding of any kind (HR, 0.71; 95% CI, 0.68–0.75).15,16
In ARISTOTLE, 81.5% and 83.1% of patients reported an adverse event in the apixaban and warfarin groups, respectively.16 Of these, more than one third of events in both groups were classified as serious. The most common (>1%) nonbleeding serious adverse events reported in patients taking apixaban during ARISTOTLE included AF (3.3%) and pneumonia (2.2%). Liver function test (AST and ALT) results higher than 3 times the upper limit of normal (ULN), accompanied by elevated bilirubin levels higher than 2 times ULN, occurred in 0.3% and 0.4% of patients taking apixaban and warfarin. Larger increases in AST/ALT were observed, albeit less frequently.16
A similar subset of patients in both groups (7.6% in the apixaban group vs. 8.4% in the warfarin group) discontinued therapy due to adverse events during ARISTOTLE.16
DRUG INTERACTIONS
Apixaban is a substrate for CYP3A4; therefore, concomitant administration of potent CYP3A4 inhibitors or inducers may affect apixaban concentration. Coadministration of apixaban with a single dose of 400 mg ketoconazole, a strong CYP3A4 (and P-glycoprotein) inhibitor, has been studied.17 The area under the curve for apixaban increased 2-fold, whereas the maximal concentration increased 1.6-fold.11 It is likely that coadministration of apixaban with a strong inhibitor of both CYP3A4 and P-glycoprotein (-azole antifungals, human immunodeficiency virus [HIV] protease inhibitors) will not be recommended. However, combined use with less-potent CYP3A4 or P-glycoprotein inhibitors (e.g., diltiazem) may be acceptable. Also of note, a drug interaction study demonstrated that coadministration of apixaban 20 mg once daily for 10 days, together with digoxin, had no effect on the pharmacokinetic profile of digoxin.18
Coadministration of apixaban with aspirin and with clopidogrel has also been studied.17 Apixaban 5 mg twice daily with aspirin 325 mg daily (in 17 healthy subjects) or apixaban 5 mg once or twice daily with 75 mg clopidogrel (in 35 healthy subjects) did not result in changes in INR or activated partial thromboplastin time. Moreover, during these studies, no major bleeding events were observed.17 Currently, limited data regarding possible interactions between apixaban and nonsteroidal anti-inflammatory drugs and between apixaban and other anticoagulants, including heparin, low-molecular-weight heparin, and glycoprotein IIb/IIIa inhibitors, are available.17 However, an additive anticoagulant effect and increased bleeding is clinically possible whenever antiplatelet or anticoagulant agents with different mechanisms of action are administered together.
Finally, due to the absence of ionizable groups in apixaban's structure, it may not be affected by medications that alter gastric pH.17
DOSING AND ADMINISTRATION
Apixaban has not yet received FDA approval. On the basis of clinical trials, however, apixaban 5 mg twice daily or 2.5 mg twice daily has been effective in patients with AF and at least one additional risk factor for stroke or who have been deemed unsuitable for warfarin therapy.15,16 The lower dose of 2.5 mg was specific to patients with 2 or more of the following criteria: age at least 80 years, body weight 60 kg or less, and serum creatinine level at least 1.5 mg/dL. Apixaban can be administered with or without food. Drug interactions mediated through CYP3A4 and P-glycoprotein are anticipated, particularly with strong inhibitors of both CYP3A4 and P-glycoprotein such as -azole antifungals (eg, ketoconazole, itraconazole, voriconazole, posaconazole), HIV protease inhibitors (eg, ritonavir), and strong CYP3A4 and P-glycoprotein inducers (eg, rifampicin, phenytoin, carbamazepine, phenobarbital, St. John's wort).12
FORMULARY CONSIDERATIONS
Although apixaban is not yet FDA approved, it represents a promising alternative to warfarin therapy in patients with NVAF who meet the clinical criteria set in the trials that have been conducted to date. Compared with aspirin or warfarin, apixaban seems to be more effective in reducing stroke and systemic emboli, and it does not seem to increase bleeding risk beyond that conveyed by aspirin or warfarin alone. The need for regular laboratory monitoring is eliminated with apixaban, and the propensity for drug interactions is anticipated to be less than that of warfarin. Apixaban dose adjustments may be necessary for patients who meet specific age, renal function, or weight criteria. The cost of apixaban is not yet known, and it probably will be more expensive than aspirin or warfarin, which are available as generic formulations.
Dr Sobieraj is assistant professor of pharmacy practice, University of Connecticut School of Pharmacy, Storrs, Conn. Dr Coleman is associate professor of pharmacy practice, University of Connecticut School of Pharmacy, Storrs, Conn.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, medical editor, University of Connecticut/Hartford Hospital, Evidence-based Practice Center, Hartford, Conn., and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn.; and by Craig I. Coleman, PharmD, associate professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
EDITORS' NOTE: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
REFERENCES
1. Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209.
2. Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9(5):348–356.
3. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Int Med 2007;146:857–67
4. Cios DA, Baker WL, Sander SD, Phung OJ, Coleman CI. Evaluating the impact of study-level factors on warfarin control in U.S.-based primary studies: a meta-analysis. Am J Health Syst Pharm. 2009;66:916–925.
5. Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm. 2009;15:244–252.
6. Eliquis. [Package insert] European Medicines Agency. Bristol-Myers Squibb/Pfizer EEIG (United Kingdom).
7. Frost C, Yu Z, Nepal S, et al. Apixaban, a direct factor Xa inhibitor: single-dose pharmacokinetics and pharmacodynamics of an intravenous formulation [abstract 142]. J Clin Pharmacol. 2008;48:1132.
8. Frost C, Yu Z, Nepal S. Food does not affect the pharmacokinetics of apixaban, an oral factor Xa inhibitor. IXth World Conference on Clinical Pharmacology and Therapeutics; July 27-August 1, 2008; Quebec City, Canada [abstract]. Can J Clin Pharmacol. 2008;15:e420–e781.
9. Frost C, Yu Z, Moore K, et al. Apixaban, an oral direct factor Xa inhibitor: multiple-dose safety, pharmacokinetics, and pharmacodynamics in healthy subjects. XXIst ISTH Congress; August 2007 [abstract]. J Thromb Haemost. 2007;5 Supplement 2:P-M–664.
10. Raghavan N, Frost CE, Yu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos. 2009;37:74–81.
11. Eriksson BI, Quinlan DJ, Weitz JI. Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor Xa inhibitors in development. Clin Pharmacokinet. 2009;48:1–22.
12. Nutescu E, Chuatrisorn I, Hellenbart E. Drug and dietary interactions of warfarin and novel oral anticoagulants: an update. J Thromb Thrombolysis. 2011;31:326–343.
13. Wang L, Zhang D, Raghavan N, et al. In vitro assessment of metabolic drug-drug interaction potential of apixaban through cytochrome P450 phenotyping, inhibition, and induction studies. Drug Metab and Dispos. 2010;38:448–458.
14. He K, He B, Grace JE, et al. Preclinical pharmacokinetics and metabolism of apixaban, a potent and selective factor Xa inhibitor [abstract]. Blood. 2006;108:910.
15. Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. New Engl J Med. 2011;364:806–817.
16. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. New Engl J Med. 2011;365:981–992.
17. Carreiro J, Ansell J. Apixaban, an oral direct factor Xa inhibitor: awaiting the verdict. Expert Opin Investig Drugs. 2008;17:1937–1945.
18. Frost C, Lee L, Li L, Nepal S, Shenker A, Reeves RA. Apixaban does not affect the pharmacokinetics of digoxin. J Clin Pharm. 2007;47:1183 [abstract 60].
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
