- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Aprotinin may increase risk in cardiac surgery; FDA issues alert
The serine protease inhibitor aprotinin (Trasylol, Bayer) may increase the risk of renal failure, myocardial infarction, heart failure, stroke, and encephalopathy among patients undergoing coronary artery bypass graft (CABG) surgery, according to an observational study in the New England Journal of Medicine (NEJM).
The serine protease inhibitor aprotinin (Trasylol, Bayer) may increase the risk of renal failure, myocardial infarction, heart failure, stroke, and encephalopathy among patients undergoing coronary artery bypass graft (CABG) surgery, according to an observational study in the New England Journal of Medicine (NEJM).
"The association between aprotinin and serious end-organ damage indicates that continued use is not prudent," the authors stated.
The study's authors suggested the antifibrinolytic therapies aminocaproic acid and tranexamic acid as alternatives to aprotinin. These therapies have not been FDA-approved for managing cardiac surgery patients.
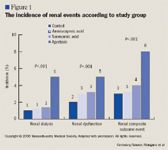
In the study recently published by the NEJM, 4,374 patients (3,477 men and 897 women aged 18 y and older) undergoing revascularization by primary or complex coronary artery surgery were divided into a control group (n=1,374) and an antifibrinolytic group (n=3,000). The antifibrinolytic group was subdivided into patients who received aprotinin (n=1,295), aminocaproic acid (n=883), or tranexamic acid (n=822). Researchers measured more than 200 covariates per patient.
By using propensity to adjust the findings of their multivariable logistic regression, the authors found that in those undergoing primary surgery, aprotinin almost doubled the odds of a renal event-renal dysfunction or renal failure requiring dialysis-compared with the control group (OR=1.89; 95% CI, 1.01–3.55; P=.04).
Other findings of the regression analysis included an increased risk of death with aprotinin (2.8% vs 1.3%, P=.02), cardiovascular events (20.4% vs 13.2%, P<.001), and cerebrovascular events (4.5% vs 1.6%, P<.001) among those undergoing primary surgery. Amino-caproic acid and tranexamic acid were not associated with these events.
Among patients who presented for complex coronary artery surgery, aprotinin also was associated with an increased number of renal events (OR=2.79; 95% CI, 1.44–5.44; P=.002) after propensity adjustment.
Relationship correlations were found between the size of the aprotinin dose and the control group. With a high dose (loading dose=2 million kalikrein-inhibitor units [KIU], total dose >4 million KIU), a significant relationship was found between renal events, cardiovascular events, and composite outcome events (P<.001) when compared with the control group. With a low dose (loading dose=1 million KIU, total dose >2 million KIU), a significant relationship was found between renal events (P<.001) and composite outcome events (P=.003) when compared with the group that received no antifibrinolytic therapy. A pair comparison between high and low doses of aprotinin found no significant relationship except with renal events (P<.001). The authors found that all 3 antifibrinolytic treatments reduced the loss of blood in patients to similar degrees (all P<.001).
Accompanying editorials praised the phase 4 study.
"The importance of this study goes beyond the specific findings of the use of aprotinin to limit blood loss in patients undergoing cardiac surgery," stated Gus J. Vlahakes, MD, in one editorial. "Some surgeons and anethesiologists who use the drug have been concerned about its potential risks. Although this observational study was not a randomized trial, the incorporation of a large number of patients gives it credibility."
In another editorial, David Hunter, MD, BS, professor of epidemiology at the Harvard School of Public Health, Boston, said the NEJM study may serve as a model.
"Studies such as the one by Mangano and colleagues point the way to the prospective design of studies to assess drug safety," Dr Hunter stated.
In response to the study in NEJM, Bayer, the drug's manufacturer, cited prospective, randomized, double-blind, placebo-controlled studies of nearly 6,500 open-heart surgery patients that the company has conducted.

SOURCES Hunter, D. First, gather the data. N Engl J Med. 2006;354;4:329–331.
Mangano D, Tudor I, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354;4:353–365.
Vlahakes, G. The value of phase 4 clinical testing. N Engl J Med. 2006;354;4:413–415.
Karkouti K, Beattie WS, Dattilo K, et al. A propensity score case-control comparison of aprotinin and tranexamic acid in high-transfusion-risk cardiac surgery.Transfusion. 2006;46;3:327–338.
Press release. Bayer clinical trials on Trasylol not consistent with New England Journal of Medicine study. Available at http:// http://www.news.bayer.com/baynews/baynews.nsf/ID/±56DA267D894364C125710300317007?Open&ccm=000. Accessed March 31, 2006.
FDA Web page. Aprotinin (marketed as Trasylol information). Available at http:// http://www.fda.gov/cder/drug/infopage/aprotinin/default.htm. Accessed February 10, 2006.
FDA Approves Combination Therapy for Pulmonary Arterial Hypertension
March 25th 2024J&J’s Opsynvi is single-tablet combination of macitentan, an endothelin receptor antagonist, and tadalafil, a PDE5 inhibitor. It will be priced on parity with Opsumit, which is also a J&J product to treat patients with PAH.
FDA Issues Complete Response Letter for Onpattro in Heart Failure Indication
October 9th 2023Alnylam Pharmaceuticals will no longer pursue this indication of Onpattro and will instead on focus on a label expansion for Amvuttra, which is in phase 3 development to treat patients with cardiomyopathy of ATTR amyloidosis.
