- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
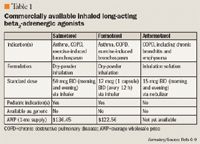
Arformoterol: The first nebulized long-acting beta2-adrenergic agonist
Bronchodilators play an important role in the management of stable chronic obstructive pulmonary disease (COPD). Although bronchodilators do not prevent the decline in lung function in patients with COPD, their efficacy in improving disease-related symptoms, reducing the frequency and severity of disease exacerbations, and improving patients' quality of life has been demonstrated in clinical trials. Arformoterol, the (R,R)-enantiomer of the selective beta2-agonist formoterol, is a potent, highly specific, nebulized long-acting beta2-adrenergic agonist recently approved by FDA for the long-term maintenance treatment of bronchoconstriction in patients with COPD, including chronic bronchitis and emphysema. In 2 large, 12-week, phase 3 studies, arformoterol demonstrated an efficacy superior to that of placebo and comparable to that of salmeterol in patients with COPD. In these trials, arformoterol was well tolerated, with a safety profile similar to that of other inhaled long-acting beta2-agonists when used at..
Dr Abdelghany is adjunct assistant professor of pharmacy practice, School of Pharmacy, University of Connecticut, Storrs, Conn, and coordinator, Investigational Drug Service, Yale-New Haven Hospital, New Haven, Conn. Dr Merl is a pharmacy practice resident, Yale-New Haven Hospital.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
Abstract

Chronic obstructive pulmonary disease (COPD) is a progressive disease characterized by airflow limitation that is not fully reversible. It is the fourth-leading cause of death in the United States and is projected to become the third-leading cause of death by 2020.1 COPD is a major economic burden in the United States, with more than $37 billion spent directly on COPD healthcare expenses and indirectly on COPD morbidity and mortality expenses in 2004.1
COPD is considered incurable with the currently available treatment options. None of the existing therapies has been demonstrated to prevent the decline in lung function associated with this disease. Currently, the goals of therapy are to prevent or provide relief of symptoms, reduce the frequency and attenuate the severity of exacerbations, prevent disease progression, and maintain or improve quality of life.2,3
Bronchodilators are considered first-line maintenance therapy in patients with COPD. Long-acting bronchodilators, including long-acting beta2-adrenergic agonists and inhaled anticholinergic drugs, are more effective than short-acting bronchodilators.4 Long-acting beta2-adrenergic agonists exert their beneficial effect by a reduction in airflow resistance with improvement in lung emptying, which leads to a reduction in resting and dynamic hyperinflation during exercise and improved exercise performance.4 The efficacy of long-acting beta2-agonists in patients with COPD has been demonstrated in several clinical trials. These agents have been demonstrated to decrease daytime and nighttime symptoms and to improve quality of life.5
CHEMISTRY AND PHARMACOLOGY
Formoterol enantiomers demonstrate different, possibly contradictory biologic activities. Arformoterol, the (R,R)-enantiomer, is 1,000-fold more potent than the (S,S)-enantiomer, demonstrates 2-fold greater potency than racemic formoterol, and may account exclusively for the activation of beta2 receptors by the racemic mixture.11 However, (S,S)-formoterol is not biologically inert. It inhibits the beneficial effects of (R,R)-formoterol on proliferation, anti-inflammatory cellular surface marker expression, and cytokine secretion. Results from studies in healthy volunteers have indicated that racemic formoterol may have proinflammatory properties that would mask the beneficial effects associated with beta2-agonists.12 Further studies are needed to determine the clinical significance of these properties and to elucidate the advantages of arformoterol over formoterol, if any.
PHARMACOKINETICS

CLINICAL TRIALS


The primary efficacy end point was postdose bronchodilation as measured by the percent change from baseline FEV1 at the end of the dosing interval (12 hours after evening dose for the BID groups; 24 hours after daily dose for the QD group) over the 12 weeks of treatment. Treatment with arformoterol or salmeterol led to a statistically significant improvement in morning trough FEV1 over 12 weeks. In both trials, the mean differences of the percent change in morning trough FEV1 with arformoterol 15 mcg BID, arformoterol 25 mcg BID, arformoterol 50 mcg QD, and salmeterol 42 mcg BID versus placebo after the first dose at Week 0 were 15.3 (95% CI, 12.1–18.4), 19.1 (95% CI, 15.8–22.3), 13.4 (95% CI, 10.2–16.6), and 14.6 (95% CI, 11.5–17.7), respectively. Improvement in morning trough FEV1 was maintained at Week 12; the mean differences of percent change in morning trough FEV1 with arformoterol 15 mcg BID, arformoterol 25 mcg BID, arformoterol 50 mcg QD, and salmeterol 42 mcg BID versus placebo at Week 12 were 9.9 (95% CI, 5.9–14.0), 13.1 (95% CI, 8.7–17.5), 10.6 (95% CI, 6.3–15.0), and 10.8 (95% CI, 7.0–14.5), respectively.6,14
The secondary efficacy end point was the percent change from baseline in 12-hour FEV1 AUC (% change in FEV1 AUC0–12 h) over 12 weeks. The mean differences of the percent change in FEV1 AUC0–12 h with arformoterol 15 mcg BID, arformoterol 25 mcg BID, arformoterol 50 mcg QD, and salmeterol 42 mcg BID versus placebo at Week 12 were 5.9 (95% CI, 3.8–7.9), 5.0 (95% CI, 2.9–7.2), 12.7 (95% CI, 10.3–15.1), and 1.7 (95 % CI, –0.1 to 3.6), respectively.
In these trials, arformoterol doses of 25 mcg BID and 50 mcg QD did not provide sufficient additional benefits on a variety of end points to warrant use of these higher doses. Therefore, a total daily dose >30 mcg is not recommended.

The median time to onset of bronchodilation after the first 15-mcg dose of arformoterol was 6.7 minutes when bronchodilation was defined as an increase in FEV1 of 15% and was 20 minutes when bronchodilation was defined as an increase in FEV1 of 12% and 200 mL. Peak bronchodilator effect generally occurred within 1 to 3 hours of dosing.6,14
Analyses of responders, defined as patients achieving a change from baseline FEV1 of ≥10% or ≥15%, were performed. Patients in all active treatment groups demonstrated superior responses compared with patients in the placebo group at Weeks 0 and 12. Among those treated with arformoterol 15 mcg BID, a ≥10% improvement in FEV1 was achieved in 93% of patients after the first dose and in 84% of patients after 12 weeks of treatment. Among those treated with salmeterol 42 mcg BID, 90% achieved a ≥10% improvement in FEV1 after the first dose, and 62% achieved a ≥10% improvement after 12 weeks of treatment. Among those treated with arformoterol 15 mcg BID, a ≥15% improvement in FEV1 was achieved in 82% of patients after the first dose and in 63% of patients after 12 weeks of treatment.14
