- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Bifeprunox: A partial dopamine-receptor agonist for the treatment of schizophrenia
Schizophrenia is a chronic psychiatric disorder that affects an estimated 1% of the population. This disorder may be treated with typical (first-generation) or atypical (second-generation) agents; a recognized concern regarding these agents is that long-term use has been associated with increased risks of serious side effects, either neurologic or metabolic in nature. Bifeprunox is a partial dopamine-receptor agonist under investigation for the treatment of patients with schizophrenia.If approved, bifeprunox may serve as an additional option for the acute and maintenance treatment of schizophrenia.

Key Points
Abstract
Schizophrenia is a chronic psychiatric disorder that affects an estimated 1% of the population. This disorder may be treated with typical (first-generation) or atypical (second-generation) agents; a recognized concern regarding these agents is that long-term use has been associated with increased risks of serious side effects, either neurologic or metabolic in nature. Bifeprunox is a partial dopamine-receptor agonist under investigation for the treatment of patients with schizophrenia. As a partial dopamine-receptor agonist, bifeprunox acts as a dopamine-system stabilizer. This proposed mechanism of action is similar to that of aripiprazole but different from that of the other currently marketed antipsychotic medications. Available clinical and safety data are limited but describe positive effects in treating acute psychotic symptoms and prolonging time to deterioration, with a generally tolerable side-effect profile. If approved, bifeprunox may serve as an additional option for the acute and maintenance treatment of schizophrenia. (Formulary. 2007;42:371–377.)
Schizophrenia is a psychotic disorder in which the usual connections among thoughts, feelings, perceptions, and behaviors are disrupted. The disorder is a chronic illness that imparts a sense of disconnection from reality and causes considerable disability in affected individuals. An estimated 1% of the general population may meet the criteria for schizophrenia diagnosis; diagnosis assumes some variation in the clinical presentation of symptoms.1
Traditional first-generation (typical) antipsychotic medications (eg, chlorpromazine, fluphenazine, haloperidol) are dopamine-receptor antagonists. This mechanism of action is effective in treating the "positive" symptoms of schizophrenia (eg, hallucinations, delusions), which are thought to be the result of dopamine hyperactivity in the brain. However, the "negative" symptoms (eg, affective flattening, decreased speech and motivation) and cognitive impairment also associated with schizophrenia are either unaffected or worsened by these drugs. The newer second-generation (atypical) antipsychotics (eg, clozapine, risperidone, olanzapine, quetiapine, ziprasidone, aripiprazole) affect dysregulated systems through mechanisms other than selective dopamine-receptor antagonism and have been demonstrated to be more efficacious in treating the negative and cognitive symptoms of schizophrenia in addition to the positive symptoms.2

Bifeprunox (Solvay Pharmaceuticals/ Wyeth) is an antipsychotic under investigation for the treatment of patients with schizophrenia. An NDA for this drug was submitted to FDA in October 2006 for review.
CHEMISTRY AND PHARMACOLOGY

PHARMACOKINETICS
The pharmacokinetic parameters of bifeprunox were reported in a pooled analysis of 21 clinical pharmacology studies in healthy adults who were treated with single (0.5 mg/d) or multiple (20–40 mg/d) bifeprunox doses.7 Absorption of bifeprunox after oral administration was demonstrated to be relatively rapid, with a median time to peak plasma concentration (Tmax) of approximately 2 hours. Relative bioavailability was estimated to be 54%, based on assessments of hepatic blood flow and oral clearance. Area under the plasma concentration-time curve over 24 hours (AUC0–24 h) was demonstrated to be dose proportional in adults receiving 20 or 40 mg/d (mean AUC0–24 h for 20 mg/d, 381.5 ng•h/mL; mean AUC0–24 h for 40 mg/d, 825.1 ng•h/mL). The effects of co-administration of bifeprunox with a high-fat meal were examined in adults treated with 40 mg/d. Tmax was delayed by 1.5 hours, maximum bifeprunox concentration (Cmax) was increased by 10%, and AUC0–24 h was increased by 29%. The authors stated that, based on these results, bifeprunox could be taken with or without food.7 The apparent volume of distribution of bifeprunox was demonstrated to be 1,300 L, a value that implies widespread distribution into peripheral tissues. In contrast, neither the parent drug nor its metabolites were distributed extensively into erythrocytes. Bifeprunox was demonstrated to be highly bound to circulating plasma proteins (>99%) in both healthy adults and in patients with hepatic or renal impairment.7 After multiple-dose administration of bifeprunox, the mean steady-state elimination half-life was 14.4 hours. Steady-state bifeprunox levels were achieved within 2 to 4 days. After single-dose (0.25 mg) oral administration of 14C-bifeprunox, 87% of the radioactive substance was excreted (13% through the urine, 74% through the feces), mostly in the form of metabolites.7
In vitro studies have demonstrated that bifeprunox primarily undergoes hepatic metabolism through the CYP2C9 and CYP3A4 enzyme pathways, with the CYP2D6 pathway acting as a minor alternative to clearing the drug through the liver. In contrast, aripiprazole metabolism is mediated primarily by CYP2D6 and CYP3A4.8 Examination of bifeprunox metabolism in adults with reduced activity of CYP2C9, an isozyme subject to genetic polymorphism, demonstrated increases in Cmax of 3.1-fold (for adults with intermediate metabolism) and 3.9-fold (for adults with slow metabolism) compared with Cmax values obtained in adults with normal metabolism. Similarly, AUC0–24 h values were elevated in this subgroup (3.7- and 7.1-fold higher for adults with intermediate metabolism and adults with slow metabolism, respectively, than for adults with normal metabolism).7
Differences in age, sex, body weight, or race did not have any clinically relevant effects on the pharmacokinetics of bifeprunox.7
CLINICAL TRIALS
To date, much of the efficacy data regarding the use of bifeprunox in the treatment of schizophrenia have been presented in posters at the American College of Neuropsychopharmacology 45th Annual Meeting (2006) and at the 2007 International Congress on Schizophrenia Research. Dose-finding studies generally used a randomized, double-blind, placebo-controlled, active-comparator-referenced, parallel-group design and enrolled patients with active symptoms of schizophrenia, as determined by criteria established in the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (text revision).9
Efficacy studies in patients with acute exacerbations of schizophrenia. In a 6-week, randomized trial, patients with acute exacerbations of schizophrenic symptoms were randomized to treatment with doses of bifeprunox 5 mg/d (n=115), 10 mg/d (n=120), or 20 mg/d (n=115); risperidone 6 mg/d (n=120); or placebo (n=119). Risperidone was included as an active reference for assay sensitivity. Bifeprunox was titrated from a starting dose of 0.125 mg on Day 1; the dose was approximately doubled every day until the target dose of 5 mg (Day 6), 10 mg (Day 7), or 20 mg (Day 8) was reached. The primary efficacy measure was the change in the total score obtained on Positive and Negative Symptom Scale (PANSS) ratings, with analyses including the last observation carried forward (LOCF).10 The PANSS is a clinician-administered instrument that has been validated for assessment of symptomatic progression in patients with schizophrenia and is considered a "gold standard" tool in clinical drug trials. A higher score on the PANSS reflects greater psychopathology.
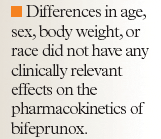

Olanzapine has also been used as an active reference drug in a bifeprunox efficacy study evaluating the treatment of acute symptoms in patients with schizophrenia.12 In a 6-week trial that randomized patients to regimens of bifeprunox 20 mg/d (n=154) or 30 mg/d (n=150), olanzapine 15 mg/d (n=150), or placebo (n=150), the primary outcome assessed was change in PANSS total score. The end point analysis demonstrated that although bifeprunox 20 and 30 mg/d resulted in improvements of PANSS total score (mean changes, –13.8 and –13.1 points, respectively), neither dose was demonstrated to be significantly superior to placebo (mean change, –10.7 points). Olanzapine, with a mean change at last assessment of –22.0 points, demonstrated statistically superior improvement compared with placebo. This study reflected an unusually high placebo response rate that the authors could not explain.12