- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Clevidipine: An intravenous dihydropyridine calcium-channel blocker for the treatment of acute hypertension
Clevidipine is an intravenous (IV) dihydropyridine calcium-channel blocker (CCB) that is approved for the reduction of blood pressure (BP) when oral therapy is not feasible or not desirable. Clevidipine is effective at reducing BP in the emergency room (ER), intensive care unit (ICU), and in the pre-, peri-, and postoperative settings.

Key Points
Abstract
Clevidipine is an intravenous (IV) dihydropyridine calcium-channel blocker (CCB) with a rapid onset of action (2–4 min) and a short duration of action (5–15 min). This agent is approved for the reduction of blood pressure (BP) when oral therapy is not feasible or not desirable. Clevidipine is effective at reducing BP in the emergency room (ER), intensive care unit (ICU), and in the pre-, peri-, and postoperative settings, with approximately 90% of treated patients achieving their systolic BP (SBP) goals regardless of treatment setting. Direct comparisons of clevidipine with nitroglycerin, sodium nitroprusside, and nicardipine in the perioperative setting have demonstrated similar mortality and cardiovascular outcomes 30 days after surgery. Clevidipine is generally well tolerated; the most commonly reported adverse events in clinical trials were headache, nausea, and vomiting. (Formulary. 2009;44:102–107.)
A rapid and severe elevation in blood pressure (BP) is referred to as acute hypertension and may lead to a life-threatening hypertensive crisis.1 Patients with systolic blood pressure (SBP) >180 mmHg or diastolic blood pressure (DBP) >120 mmHg are considered as having a hypertensive crisis, which can be further classified into either hypertensive urgency (when evidence of target organ damage is lacking) or hypertensive emergency (when evidence of target organ damage requiring immediate BP reduction is present).2 Organs most likely affected by hypertensive emergencies include the brain, heart, kidneys, and blood vessels.3 Because of the risk of serious adverse events such as stroke, myocardial infarction (MI), heart failure, and renal failure, hypertensive emergencies must be managed immediately with parenteral antihypertensive therapy.3
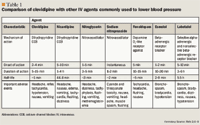
CHEMISTRY AND PHARMACOLOGY
Clevidipine (butyroxymethyl methyl 4-[2',3'-dichlorophenyl]-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate) is insoluble in water and thus is available as an oil-in-water emulsion.6 This agent is a dihydropyridine L-type CCB that mediates the influx of calcium in arterial smooth muscle, thereby reducing BP by arterial dilation.10 Clevidipine has no effect on cardiac filling pressure and does not affect venous circulation.6
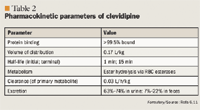
PHARMACOKINETICS
CLINICAL TRIALS
Clevidipine has been evaluated in multiple randomized, placebo- and active-controlled trials for the treatment of acute hypertension occurring before, during, or after cardiac surgery. In addition, preliminary data from a noncontrolled trial of acute hypertension in the emergency room (ER) or intensive care unit (ICU) are also available.
Placebo-controlled trials. The Efficacy Study of Clevidipine Assessing its Preoperative Antihypertensive Effect in Cardiac Surgery-1 (ESCAPE-1) was a randomized, double-blind, placebo-controlled trial that assessed the safety and efficacy of clevidipine compared with placebo during the immediate preoperative period.12 Patients experiencing hypertension before cardiac surgery who had a history of hypertension within the previous 6 months were randomized to treatment with either clevidipine or placebo. After randomization, patients (N=105) received the study drug only if their SBP was ≥160 mmHg and if they required an SBP reduction of ≥15% from baseline. A continuous IV infusion of clevidipine 0.4 mcg/kg/min was initiated when elevated SBP was detected; the dosage was then titrated to effect or to a maximum dose of 8 mcg/kg/min for a total of 30 to 60 minutes or until induction of anesthesia. The primary outcome was incidence of treatment failure, defined as premature and permanent discontinuation of study drug for any reason or failure to reach target SBP reduction (≥15% reduction from baseline), thus requiring use of alternative antihypertensive therapy. The time to target SBP was also assessed.
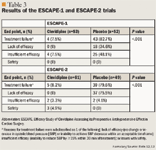
The Efficacy Study of Clevidipine Assessing its Preoperative Antihypertensive Effect in Cardiac Surgery-2 (ESCAPE-2) compared the safety and efficacy of clevidipine versus placebo in the immediate postoperative period.13 Patients experiencing hypertension after cardiac surgery were randomized to treatment with either clevidipine or placebo. After randomization, patients (N=110) received clevidipine or placebo only if their SBP was ≥140 mmHg within 4 hours after arriving at the postoperative setting and if they required an SBP reduction of ≥15% from baseline. As in the ESCAPE-1 trial, a continuous IV infusion of clevidipine 0.4 mcg/kg/min was initiated, and the dosage was then titrated to effect or to a maximum dose of 8 mcg/kg/min and administered for a total of 30 to 60 minutes. The primary outcome was treatment failure, defined as premature and permanent discontinuation of study drug for any reason or failure to reach target SBP reduction (≥15% reduction from baseline), thus requiring use of alternative antihypertensive therapy. Time to reach target SBP was also evaluated.
Among patients who received study drug in the modified intent-to-treat population, those treated with clevidipine had a significantly lower rate of treatment failure versus those treated with placebo (P<.0001) (Table 3).13 Clevidipine-treated patients reached their target SBP in a median time of 5.3 minutes (95% CI, 4–7 min), whereas the median time could not be evaluated in placebo-treated patients because most patients did not achieve this end point.
Active-controlled trials. The Evaluation of Clevidipine in the Perioperative Treatment of Hypertension Assessing Safety Events (ECLIPSE) trials were 3 open-label, randomized studies that compared clevidipine with nitroglycerin, sodium nitroprusside, and nicardipine in patients aged ≥18 years who were experiencing hypertension around the time of scheduled cardiac surgery.14 After randomization, patients received study drug only if it was deemed clinically necessary to use an antihypertensive agent based on physician discretion and institutional guidelines. Study drug was titrated until a target BP was attained, and treatment was continued until discharge from the ICU. A continuous IV infusion of clevidipine 0.4 mcg/kg/min was initiated, with the dosage then force-titrated by a doubling of the dose every 90 seconds up to 3.2 mcg/kg/min; the dosage was then titrated based on clinical response. Nitroglycerin, sodium nitroprusside, and nicardipine were dosed based on usual practice outlined in institutional guidelines. Clevidipine was compared with both nitroglycerin and sodium nitroprusside before, during, and after surgery, whereas clevidipine was compared with nicardipine only after surgery because nicardipine is typically used only in the postoperative setting. The primary end point was the incidence of death, stroke, MI, and renal dysfunction within 30 days after surgery. The secondary end point was assessed with an area-under-the-curve (AUC) analysis to capture the magnitude (mmHg) and time (min) of BP levels beyond the therapeutic range, normalized per hour, and presented as AUCSBP-D in units of mmHg•min/h.
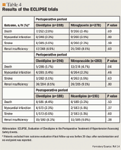
The pooled clevidipine population also demonstrated better BP control compared with the pooled active-control population, as evidenced by lower median AUCSBP-D values (3.79 vs 7.79 mmHg•min/h; P=.0004). Comparing clevidipine with each agent individually, investigators observed that there was significantly greater BP control with clevidipine versus nitroglycerin (median AUCSBP-D, 4.14 vs 8.87 mmHg•min/h; P=.0006) and versus sodium nitroprusside (median AUCSBP-D, 4.37 vs 10.50 mmHg•min/h; P=.003), but BP control was comparable for clevidipine versus nicardipine (median AUCSBP-D, 1.76 vs 1.69 mmHg•min/h; P=.85).
Noncontrolled trial. The Evaluation of the Effect of Ultra-Short-Acting Clevidipine in the Treatment of Patients with Severe Hypertension (VELOCITY) trial was a prospective, open-label, noncontrolled trial evaluating the safety and efficacy of clevidipine in patients who presented in the ER or ICU with severe hypertension (SBP >180 mmHg or DBP >115 mmHg).15 Evidence of target organ damage was neither an inclusion nor exclusion criterion in the trial. Enrolled patients (N=131) were clinically evaluated and were given a patient-specific initial target SBP range based on physician discretion, taking into consideration the presenting condition, baseline BP, and patient comorbidities. A continuous IV infusion of clevidipine 2 mg/h was administered for at least the first 3 minutes of therapy, and the dosage was then titrated within the next 30 minutes by doubling the dose every 3 minutes until the patient's initial target SBP was achieved, up to a maximum dose of 32 mg/h. After the first 30 minutes of clevidipine infusion, the SBP target range could be altered based on physician discretion to meet long-term BP goals. Clevidipine was continued for durations of 18 to 96 hours, with the dose adjusted as necessary to maintain target SBP. After 18 hours, oral antihypertensive therapy could be used if appropriate, but treatment had to be initiated 1 hour before the anticipated end of clevidipine therapy. The primary efficacy end point was the proportion of patients achieving their initial target SBP within 30 minutes of clevidipine initiation. The primary safety end point was the proportion of patients whose SBP fell below the lower limit of the initial target SBP range within 3 minutes of clevidipine initiation. The success rate of oral therapy transition, defined as the proportion of patients with SBP remaining within the target range 6 hours after discontinuation of clevidipine, was also assessed as a secondary end point.
Patients included in the primary efficacy analysis were those who had SBP above their initial target range at the time of clevidipine initiation (modified intent-to-treat population, n=117), whereas those in the safety analysis were patients who received ≥1 dose of clevidipine, regardless of SBP at the time of initiation (n=126). Of the 117 patients in the efficacy analysis, 104 (88.9%) reached their initial target SBP within 30 minutes. Within 3 minutes of initiating clevidipine, 2 out of the 126 patients (1.6%) in the safety analysis experienced SBP reductions below the lower limit of their target SBP range. For the secondary end point of oral antihypertensive transition, 115 of the 126 patients (91.3%) in the safety analysis successfully transitioned to oral antihypertensive therapy at 6 hours after discontinuation of clevidipine.
ADVERSE EVENTS
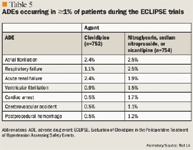
It has been suggested that the clevidipine lipid emulsion may result in increased triglyceride levels.15 In the VELOCITY trial, long-term infusion of clevidipine lipid emulsion did not alter the median percentage change in triglyceride levels, and a post-hoc analysis demonstrated that there was no relationship between clevidipine dose and change in triglyceride levels from baseline to 6 hours after clevidipine treatment (Pearson's correlation coefficient [r]=–0.0269).15 No specific data on this effect were provided in the ECLIPSE trial results, although the investigators stated that changes in triglyceride levels were similar for clevidipine and comparator agents.14

DRUG INTERACTIONS
Clevidipine and its main metabolite do not inhibit or stimulate the cytochrome P450 isoenzyme system and would therefore be unlikely to cause drug interactions through that mechanism. Unfortunately, no pharmacokinetic or pharmacodynamic clinical drug interaction studies evaluating clevidipine are available. Potential pharmacodynamic drug interactions with clevidipine might include dramatic reductions in BP when this agent is combined with other IV antihypertensive agents, as well as an increased risk of pancreatitis when clevidipine is administered with other drugs formulated as a lipid emulsion, such as propofol.16
DOSING AND ADMINISTRATION
Clevidipine is available as a single-use, ready-to-use vial that must be used within 4 hours of vial puncture. Because this agent is formulated as a lipid emulsion, clevidipine vials have a milky white appearance. Although no drug-compatibility data currently exist, precipitations would be difficult to detect; therefore, clevidipine should be not be used in the same line as other medications. Clevidipine has been demonstrated to be compatible with various IV fluids including sodium chloride 0.9%, dextrose 5%, and lactated ringers. IV clevidipine, administered via an infusion pump, is initiated at a dose of 1 to 2 mg/h and may be titrated to achieve desired BP reductions by doubling the dose as often as every 90 seconds. The rate of clevidipine titration may become less rapid as BP nears the goal, with dose increases every 5 to 10 minutes at a rate of 1 to 2 mg/h. The maximum dose evaluated in clinical trials was 32 mg/h, but this dose was used in only a few patients; most patients reached a maximum dose of 16 mg/h. When switching to oral therapy is deemed appropriate, clevidipine should be tapered to allow time for oral therapy to become therapeutic. No dosage adjustments are necessary for renal or hepatic insufficiency.6
Dr Phung is an outcomes research fellow, University of Connecticut/Hartford Hospital Evidence-Based Practice Center. Dr Baker is a senior research scientist, University of Connecticut/Hartford Hospital Evidence-Based Practice Center. Dr White is professor of Pharmacy Practice, University of Connecticut School of Pharmacy, and director, University of Connecticut/Hartford Hospital Evidence-Based Practice Center. Dr Coleman is assistant professor of Pharmacy Practice, University of Connecticut School of Pharmacy, and methods chief and program director, University of Connecticut/Hartford Hospital Evidence-Based Practice Center.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
REFERENCES
1. Chobanian AV, Bakris GL, Black HR, et al; the National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report [erratum in JAMA. 2003;290:197]. JAMA. 2003;289:2560–2572.
2. Gilmore RM, Miller SJ, Stead LG. Severe hypertension in the emergency department patient. Emerg Med Clin N Am. 2005;23:1141–1158.
3. Marik PE, Varon J. Hypertensive crises: Challenges and management [erratum in Chest. 2007;132:1721]. Chest. 2007;131:1949–1962.
4. Haas AR, Marik PE. Current diagnosis and management of hypertensive emergency. Semin Dial. 2006;19:502–512.
5. Varon J, Marik PE. Perioperative hypertension management. Vasc Health Risk Manag. 2008;4: 615–627.
6. Cleviprex [package insert]. Parsippany, NJ: The Medicines Company; 2008.
7. Cardene I.V. [package insert]. Redwood City, CA: PDL BioPharma, Inc.; 2008.
8. Nitroglycerin injection [package insert]. Shirley, NY: American Regent; 2002.
9. Nitropress [package insert]. Lake Forest, IL: Hospira, Inc.; 2006.
10. Gradman AH, Vivas Y. New therapeutic perspectives with clevidipine: An ultra-short-acting intravenous Ca2+ channel blocker. Expert Opin Investig Drugs. 2007;16:1449–1457.
11. Nordlander M, Sjöquist PO, Ericsson H, Ryden L. Pharmacodynamic, pharmacokinetic and clinical effects of clevidipine, an ultrashort-acting calcium antagonist for rapid blood pressure control. Cardiovasc Drug Rev. 2004;22:227–250.
12. Levy JH, Mancao MY, Gitter R, et al. Clevidi- pine effectively and rapidly controls blood pressure preoperatively in cardiac surgery patients: The results of the randomized, placebo-controlled efficacy study of clevidipine assessing its preoperative antihypertensive effect in cardiac surgery-1. Anesth Analg. 2007;105:918–925.
13. Singla N, Warltier D, Gandhi S, et al; the ESCAPE-2 Study Group. Treatment of acute postoperative hypertension in cardiac surgery patients: An efficacy study of clevidipine assessing its postoperative antihypertensive effect in cardiac surgery-2 (ESCAPE-2), a randomized, double-blind, placebo-controlled trial. Anesth Analg. 2008;107:59–67.
14. Aronson S, Dyke CM, Stierer KA, et al. The ECLIPSE trials: Comparative studies of clevidipine to nitroglycerin, sodium nitroprusside, and nicardipine for acute hypertension treatment in cardiac surgery patients. Anesth Analg. 2008;107:1110–1121.
15. Pollack CV, Varon J, Garrison NA, Ebrahimi R, Dunbar L, Peacock WF 4th. Clevidipine, an intravenous dihydropyridine calcium channel blocker, is safe and effective for the treatment of patients with acute severe hypertension. Ann Emerg Med. 2009;53:329–338.
16. Diprivan [package insert]. Wilmington, DE: AstraZeneca; 2005.
FDA Approves Combination Therapy for Pulmonary Arterial Hypertension
March 25th 2024J&J’s Opsynvi is single-tablet combination of macitentan, an endothelin receptor antagonist, and tadalafil, a PDE5 inhibitor. It will be priced on parity with Opsumit, which is also a J&J product to treat patients with PAH.
FDA Issues Complete Response Letter for Onpattro in Heart Failure Indication
October 9th 2023Alnylam Pharmaceuticals will no longer pursue this indication of Onpattro and will instead on focus on a label expansion for Amvuttra, which is in phase 3 development to treat patients with cardiomyopathy of ATTR amyloidosis.
