- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Crawford nominated for permanent FDA commissioner role
President George W. Bush recently nominated Lester M. Crawford, DVM, PhD, to take the permanent role of FDA commissioner after serving as acting or deputy administrator since February 2002.

Dr Crawford's nomination, which was pending Senate confirmation at press time, came a day before he and Leavitt announced the creation of a new independent Drug Safety Oversight Board (DSB). The DSB will solicit input on how FDA should disseminate emerging information prior to regulatory action. Through enhanced communication channels such as a "Drug Watch" web page and consumer-friendly publications, DSB will provide medication safety information to the public.
Dr Crawford's nomination also came just before a highly anticipated meeting of 2 FDA advisory committees focusing on the risk-benefit profiles of specific NSAIDs, especially the COX-2 inhibitors (see Medication Safety and Reliability article).
SOURCES HHS website. Statement by Mike Leavitt, secretary of Health and Human Services, regarding the nomination of Dr Les Crawford to be FDA commissioner [press release]. Available at: http:// http://www.hhs.gov/news/press/2005pres/20050214.html. Accessed February 18, 2005.
FDA website. FDA improvements in drug safety monitoring [press release]. Available at: http:// http://www.fda.gov/oc/factsheets/drugsafety.html. Accessed February 18, 2005.
POLICY IN BRIEFGeneric drug purchases, not importation, best means of lowering consumer costs Two studies conducted by HHS and the Department of Commerce concluded that legalizing prescription drug importation could drive up drug costs for US consumers and government regulatory spending. The studies also found that the purchase of generic equivalents for the 29 top-selling brand name drugs could save consumers more than $700 million. If generics were purchased in every case, savings could total $17 billion.
The HHS report stated that a policy of legalizing importation could deter pharmaceutical companies from conducting R&D, which could "result in between 4 to 18 fewer new drugs being introduced per decade at a substantial cost to society."
Although approximately 12 million prescription drug products totaling approximately $700 million were imported from Canada into the United States in 2003, and an equivalent amount was imported from other international locations, the HHS report stated that foreign government officials are reluctant to implement safeguards to ensure the safety of exported drugs.
In a letter to House Speaker Dennis Hastert (R-IL), former HHS Secretary Tommy G. Thompson and Commerce Secretary Donald L. Evans stated that if an importation program were put in place, it should: prohibit personal shipments through mail; include only drugs that will yield savings (eg, high-volume products for which a US-approved generic is not available); and require that source information on packaging meet FDA requirements.
SOURCES HHS website. HHS Report on Prescription Drug Importation. Available at: http:// http://www.hhs.gov/importtaskforce/. Accessed February 1, 2005.
Department of Commerce website. Pharmaceutical price controls in OECD countries. Available at: trade.gov/td/chemicals/drugpricingstudy.pdf. Accessed February 1, 2005.
USP submits final Medicare Drug Benefit Model Guidelines According to the United States Pharmacopeia (USP), the Medicare Part D Drug Benefit Model Guidelines provide CMS with a "science-based standard" that may be used by plans to develop formularies. USP is a nongovernmental organization that seeks to ensure the quality, consistency, and safety of medications.
USP compiled a list of 146 therapeutic categories/classes, and a separate list of 128 "formulary key drug types" that CMS can use to measure a plan's comprehensiveness. "By checking for at least 1 item in each of the formulary types, CMS can ensure that a formulary provides access to medications and will not discourage enrollment of eligible beneficiaries."
USP plans to review the guidelines periodically to include newer drugs.
SOURCE USP website. USP statement on model guidelines. Available at: http:// http://www.onlinepressroom.net/uspharm/. Accessed February 1, 2005.
Acronyms
HHS=US Department of Health & Human Services; CMS=Centers for Medicare & Medicaid Services

Peter Ashkenaz, a CMS spokesperson, said in response to the extension: "The final formulary guidelines were revised to ask that plans make a reasonable effort to review new molecular entities within 90 days and to make a final decision within 180 days. This also applies to FDA-approved indications." The guidelines further state: "Plans must make access to new drugs available to enrollees when medically appropriate via exceptions processes even before this deadline."
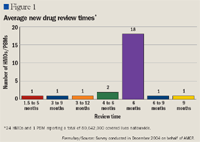
Paul N. Urick, RPh, a member of AMCP's legislative committee, conducted the survey, which found 80% of plans take 6 months or more to review new drugs. Twenty-four HMOs and 1 PBM responded, representing a total of 80 million lives (60 million and 20 million lives, respectively).
Urick said that an insufficient review period can result in long-term implications: "Clearly, the focus should remain on solid drug programs to support the elderly, and cutting the review time beyond the market standard should not derail this important cause."
Judith Cahill, executive director of AMCP, also supported a longer review time: "A comprehensive review of any new chemical entity is an exhaustive process, requiring considerable research, time, and effort. Much can be learned about patients' reactions to drugs after the tightly controlled clinical trials end and drugs are put into use by the general population."
The guidelines, stipulated by the Medicare Modernization Act, lay out a protocol for how CMS will review Medicare prescription drug benefit plans to ensure that beneficiaries receive clinically appropriate medications at the lowest possible cost.
The oversight process in the formulary guideline focuses on 3 areas:
CMS encourages plans to submit formularies that are similar to those in widespread use and have a broad distribution of categories and classes. If plans seek to offer unusual benefits, CMS may ask the plans to provide written clinical justification for these medications.
The final guidelines can be accessed at http:// http://www.cms.hhs.gov/pdps/formularyguidance.pdf.
SOURCE Academy of Managed Care Pharmacy website. AMCP supports approach taken by CMS in draft Medicare Drug Formulary Guidance [press release]. Available at: http:// http://www.amcp.org/. Accessed February 28, 2005.
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
