- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Crizotinib: A novel, targeted gene therapy for the treatment of non-small-cell lung cancer
Crizotinib is a new kinase inhibitor recently approved by FDA for the treatment of patients with locally advanced or metastatic non-small-cell lung cancer who express the anaplastic lymphoma kinase gene.

Key Points
Abstract
Crizotinib is a new kinase inhibitor recently approved by FDA for the treatment of patients with locally advanced or metastatic non-small-cell lung cancer who express the anaplastic lymphoma kinase (ALK) gene. FDA approval was based on objective response rate in 2 multicenter, single-arm studies. Crizotinib demonstrated response rates of 50% to 60% in patients enrolled in these studies-significantly better results than any previously reported in this setting. Crizotinib was well tolerated, with less than 10% of patients discontinuing due to treatment-related adverse events. Ongoing clinical trials are evaluating crizotinib's impact on survival, its role in the first-line setting, and its safety and efficacy in other malignancies. The development and accelerated approval of targeted therapies such as crizotinib represent another significant milestone in the treatment of cancer. (Formulary. 2011;46:460–472.)
Lung cancer is the second-most common malignancy and the leading cause of cancer death in both men and women in the United States, with an estimated 221,130 new cases and 156,940 deaths anticipated in 2011.1 There are 2 main types of lung cancer, small-cell lung cancer and non-small-cell lung cancer (NSCLC). NSCLC, which makes up 85% of all lung cancer cases, is further divided into 3 major types-squamous-cell carcinoma, adenocarcinoma, and large-cell carcinoma.
Despite advances in cytotoxic chemotherapy and the addition of newer cytotoxic agents, the prognosis for patients with NSCLC continues to be poor. A comparison between 4 platinum-containing regimens in patients with advanced disease showed a therapeutic plateau with a similar disease-free survival and a median survival of 7.9 months.2
Growing knowledge of molecular oncology has helped scientists identify several potential therapeutic targets, and recent efforts have been directed toward developing novel therapies that target tumors' specific proteins or gene mutations. The era of personalized medicine in lung cancer started with the discovery of the activated epidermal growth factor receptor (EGFR) mutations at exons 19 and 21. EGFR tyrosine kinase inhibitors, such as gefitinib and erlotinib, have significant single-agent activity and increase progression-free survival in patients with these mutations.3 These drugs are now recommended in the first-line setting in this selected patient population.4,5
In 2007, a fusion gene between the echinoderm microtubule-associated protein-like 4 (EML4) gene and the anaplastic lymphoma kinase (ALK) gene was identified in a subset of NSCLC patients using retrovirus-mediated expression screening.6 This fusion protein possesses transforming activity leading to promotion of cellular growth and inhibition of apoptosis that is dependent on its catalytic activity. All domains of EML4 contribute to the oncogenic potential of EML4-ALK.
Approximately 3% to 5% of NSCLC patients have tumors that are positive for the ALK fusion gene. The mutation tends to occur in younger patients, those with more advanced NSCLC, and in never or former light smokers.7 ALK inhibition is a new strategy that represents another significant advance in the treatment of NSCLC and the area of personalized therapy. Crizotinib (Xalkori, Pfizer Labs), a first-in-class ALK inhibitor, was recently approved by FDA for the treatment of patients with locally advanced or metastatic NSCLC that is ALK-positive. The drug was approved with a companion diagnostic test designed to detect rearrangements of the ALK gene. The approval was based on 2 multicenter, single-arm studies: PROFILE 1005, and Study 1001, a part 2 expansion cohort of a phase 1 study. This approval, in addition to using EGFR mutation status in treatment decisions, represents a paradigm shift in the management of NSCLC.
CHEMISTRY AND PHARMACOLOGY
Similar to other tyrosine kinase inhibitors, acquired resistance to crizotinib has been reported. Mechanisms of resistance include amplification of the EML4-ALK gene and development of a gatekeeper mutation, L1196M, within the kinase domain.11,12 Other mechanisms of crizotinib resistance will likely be discovered.
PHARMACOKINETICS
The pharmacokinetic parameters of crizotinib were evaluated in 167 patients enrolled in an open-label, multicenter phase 1 study.13 After a single dose of 250 mg, Cmax was achieved at median time to peak concentration of 4 to 6 hours. Following repeated doses of 250 mg twice a day, crizotinib plasma concentrations reached steady state within 15 days. The mean absolute bioavailability of crizotinib was 43% following a single 250-mg dose. The solubility of crizotinib decreases with increasing pH and its bioavailability may be reduced with coadministration with drugs that elevate gastric pH. Total exposure to crizotinib is also affected by food; a high-fat meal reduced crizotinib AUC∞ and Cmax by approximately 14%.14 Crizotinib seems to poorly penetrate the blood-brain barrier.15
Crizotinib is a substrate of CYP3A, a substrate of P-glycoprotein, and a moderate inhibitor of CYP3A. It is extensively metabolized by CYP3A4/5, with the main metabolic pathways being oxidation of the piperidine ring to crizotinib lactam and O-dealkylation, with subsequent phase 2 conjugation of O-dealkylated metabolites. The metabolism of a single dose of crizotinib was affected by CYP3A4 inhibitors or inducers; however the effect on steady-state exposure has not been evaluated.14
In a study in healthy subjects receiving a single 250-mg radiolabeled crizotinib dose, 63% and 22% of the administered dose was recovered in feces and urine, respectively, with approximately 53% and 2.3% of the dose recovered unchanged. Clearances decrease at steady state due to auto-inhibition of CYP3A by crizotinib after multiple dosing. The mean terminal half life after single-dose administration was 42 hours.13
Crizotinib pharmacokinetic parameters do not appear to be affected by renal impairment. Steady-state trough concentrations in patients with mild and moderate renal impairment were similar to those in patients with normal renal function.14 On the other hand, hepatic impairment is likely to increase plasma crizotinib concentrations. However, data in patients with hepatic impairment are unavailable. The pharmacokinetics of crizotinib is not affected by sex, age, race, or body weight. Cmax and AUC in Asian patients were 1.57- and 1.50-fold higher than those seen in non-Asians. However, this difference is not considered to be clinically significant.13
CLINICAL TRIALS
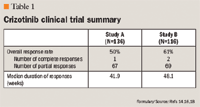
In Study A, the median duration of treatment was 22 weeks. The ORR was 50% (95% CI: 42%, 59%) in Study A with 1 complete response. Tumor response was seen during the first 8 weeks of treatment in 79% of patients. Median response duration of 41.9 weeks was observed.14,16 In Study B, the median duration of treatment was 32 weeks. The ORR was 61% (95% CI: 52%, 70%) with 2 complete responses. Tumor response was seen during the first 8 weeks of treatment, and median response duration of 48.1 weeks was observed. A preliminary median progression-free survival is reported at 10 months.18
Of note, the impact of crizotinib on overall survival was studied in crizotinib-treated patients compared with historical ALK-positive patients who were not treated with crizotinib. The 1-year survival was 77% for crizotinib-treated patients compared with 73% in the historical controls, and the 2-year survival was 64% versus 33%, respectively. Additionally, the use of crizotinib as a second- or third-line therapy improved survival compared to historical controls, with a 1-year overall survival of 71% versus 46% and 2-year overall survival of 61% versus 9%.19
DRUG INTERACTIONS
Crizotinib is extensively metabolized in the liver, specifically by the CYP3A4 and CYP3A5 enzymes. Strong CYP3A inhibitors such as ketoconazole, indinavir, and voriconazole should be avoided due to the increases in plasma concentrations of crizotinib. Moderate CYP3A4 inhibitors should be used with caution. Patients should not consume grapefruit or grapefruit juice while on crizotinib since grapefruit can inhibit CYP3A. Strong CYP3A inducers, such as phenytoin, rifampin, and St. John's wort, decrease plasma concentrations of crizotinib and should be avoided.14
Crizotinib has proven, in vivo and in vitro, to be a time-dependent inhibitor of CYP3A, therefore increasing plasma concentrations of other drugs metabolized by this enzyme system. Dose reductions or change in therapy may be necessary for medications that are predominantly metabolized by CYP3A when used concomitantly with crizotinib.14
The solubility of crizotinib depends on gastric pH, with an increase in solubility at a lower pH. The use of agents that can increase pH, such as antacids, proton pump inhibitors, and H2 antagonists, can theoretically reduce the solubility of crizotinib and its bioavailability.14
Crizotinib inhibits P-glycoprotein in vitro. Agents that are substrates of P-glycoprotein can potentially have increased plasma concentrations if co-administered with crizotinib.14
ADVERSE EVENTS
The 255 patients enrolled in Study A and Study B provide data on grades 1 to 4 crizotinib-related adverse reactions.14 In Study A, dose interruptions occurred in 36% of patients and lasted more than 2 weeks in 13% of patients. In Study B, dose interruptions occurred in 45% of patients and lasted longer than 2 weeks in 19% of patients. Crizotinib discontinuation due to treatment-related adverse events occurred in 6% of patients in Study A and 3% of patients in Study B. Vision disorder, nausea, diarrhea, vomiting, edema, and constipation were the most common adverse reactions (>25%) observed in both studies.14 In Study B, nausea, diarrhea, and vomiting were most prominent in cycle 1 and diminished over time. However, constipation became worse over time in patients enrolled in Study A. Severe adverse events (grade 3–4), which include increases in ALT, neutropenia, and dyspnea, were observed in less than 15% of patients.16,18
Grade 1 to 2 bradycardia was seen in 5% of patients; patients should be monitored for QTc prolongation if they are on medications that can prolong the QTc interval or have a history of or have predisposition to QTc prolongation. Serious adverse events leading to death observed in 2% of patients were pneumonia, dyspnea, and pulmonary embolism.14
Vision disorders were noted in 62% of patients, frequently occurring in the first 2 weeks of treatment initiation. Vision disorders present as visual impairment, photopsia, blurred vision, vitreous floaters, photophobia, and diplopia. Patients should be monitored for vision changes as they can be indicative of more serious complications such as a retinal hole or pending retinal detachment.
DOSING AND ADMINISTRATION
Crizotinib is available as 250-mg capsules and 200-mg capsules. The recommended dose of crizotinib is 250 mg taken twice daily with or without food until clinical benefit from therapy is no longer observed. Capsules should be swallowed whole. Missed doses should be skipped if the next dose is due within 6 hours.14
Crizotinib has not been studied in patients with AST or ALT greater than 2.5 times the upper limit of normal (ULN) (greater than 5 times ULN if due to liver metastases) and/or total bilirubin greater than 1.5 times ULN. It should be used with caution in patients with hepatic impairment since it is extensively metabolized in the liver. Additionally, due to lack of clinical and pharmacokinetic data, crizotinib should be prescribed with caution in patients with severe renal impairment (CrCl <30 mL/min) or patients with end-stage renal disease.14

FORMULARY CONSIDERATIONS
The approval of crizotinib represents a significant milestone in the era of personalized medicine. Although only 3% to 5% of NSCLC patients have tumors that are positive for the ALK gene rearrangement, lung cancer is the second most common malignancy and thousands of patients will likely benefit from the drug in the United States every year. Crizotinib was granted accelerated approval based on the objective response rate, which likely predicts clinical benefit. However, additional trials will be required to confirm the drug's clinical benefit and impact on overall survival in NSCLC.
The efficacy and safety of crizotinib compared to pemetrexed or docetaxel in previously treated ALK-positive NSCLC patients is under investigation in an ongoing clinical trial. Another study is comparing its efficacy and safety to pemetrexed + cisplatin or pemetrexed + carboplatin in the first-line setting. Enrollment in these trials will be affected by the commercial availability of the drug and whether insurance companies will allow oncologists to prescribe the drug for an off-label indication. Crizotinib has significant activity in anaplastic large-cell lymphoma, and a clinical trial testing its safety and efficacy in this disease is also under way.
Tumors eventually develop acquired resistance to crizotinib, and strategies to overcome crizotinib resistance will be needed for patients who have disease progression while on treatment.
Similar to other targeted therapies, crizotinib will have significant financial cost; the estimated cost per month is $9,600. This cost is well justified by avoiding the cost of other ineffective treatments in ALK-positive patients. The drug will only be available at certain specialty pharmacies, and the manufacturer has a copay assistance program for crizotinib prescriptions. Insured, eligible patients will pay no more than $100 for each prescription.
Dr Patel is an oncology clinical pharmacist, Yale-New Haven Hospital. Dr Abdelghany is adjunct assistant professor of pharmacy practice, School of Pharmacy, University of Connecticut, Storrs, Conn., and coordinator, Investigational Drug Service, Yale-New Haven Hospital, New Haven, Conn.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, medical editor, University of Connecticut/Hartford Hospital, Evidence-based Practice Center, Hartford, Conn., and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn; and by Craig I. Coleman, PharmD, associate professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
EDITORS' NOTE:The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
REFERENCES
1. American Cancer Society. Cancer Facts & Figures 2011. Atlanta, GA: 2011.
2. Schiller JH, Harrington D, Belani CP, et al, for the Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98.
3. Mok TS. Personalized medicine in lung cancer: what we need to know. Nat Rev Clin Oncol. 32 Aug 2011; doi: 10.1038/nrclinonc.2011.12.
4. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Small Cell Lung Cancer. V.1.2012. Accessed at http://www.nccn.org/professionals/physician_gls/PDF/sclc.pdf on September 20, 2011.
6. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566.
7. Sasaki T, Rodig SJ, Chirieac LR, Janne PA. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer. 2010;46:1773–1780.
8. Choi YL, Takeuchi K, Soda M et al. Identification of novel isoforms of the EML4-ALK transforming gene in non-small cell lung cancer. Cancer Res. 2008;68:4971–4976.
9. Cui JJ, Tran-Dubè M, Shen H, ét al. Structure based drug design of crizotinib (PF-02341066), a potent and sélective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem. 2011;54:6342–6363.
10. Tanizaki J, Okamoto I, Okamoto K, et al. MET tyrosine kinase inhibitor crizotinib (PF-02341066) shows differential antitumor effects in non-small cell lung cancer according to MET alterations. J Thorac Oncol. 2011;6:1624–1631.
11. Sasaki T, Koivunen J, Ogino A, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011;71:6051–6060.
12. Katayama R, Khan TM, Benes C, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci U S A. 2011;108:7535–7540.
13. Li C, Alvey C, Bello A, Wilner KD, Tan W. Pharmacokinetics (PK) of crizotinib (PF-02341066) in patients with advanced non-small cell lung cancer (NSCLC) and other solid tumors. J Clin Oncol. 2011:29(suppl);abstr e13065.
14. Prescribing Information. Xalkori (crizotinib) oral capsules. New York, NY: Pfizer Labs. August 2011.
15. Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011:29;e443–e445.
16. Crino L, Kim D, Riely GJ, et al. Initial phase II results with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC): PROFILE 1005. J Clin Oncol. 2011:29(suppl);abstr 7514.
17. Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703.
18. Camidge DR, Bang Y, Kwak EL, et al. Progression-free survival (PFS) from a phase I study of crizotinib (PF 02341066) in patients with ALK-positive non-small cell lung cancer (NSCLC). J Clin Oncol. 2011:29(suppl);abstr 2501.
19. Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011:12:1004-1012.
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
