- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Current and future applications of desirudin: A bivalent, subcutaneous direct thrombin inhibitor
Results of the PREVENT-HIT study suggest that desirudin may be a useful alternative to argatroban in the setting of heparin-induced thrombocytopenia. This article reviews the pharmacology and clinical studies of subcutaneous desirudin.
ABSTRACT
Desirudin is a bivalent direct thrombin inhibitor (DTI) indicated for the prevention of deep-vein thrombosis in patients undergoing elective hip replacement surgery. It is the only DTI approved for subcutaneous administration. Desirudin is highly selective for thrombin and binds with high affinity to both soluble and clot-bound thrombin. In patients undergoing hip replacement, desirudin is more effective than unfractionated and low-molecular-weight heparins in preventing deep-vein thrombosis, with a safety profile similar to both agents. Its safety and efficacy was recently compared with argatroban in an exploratory study in patients with suspected heparin-induced thrombocytopenia with or without thrombosis (PREVENT-HIT study). The results suggest that desirudin may be a useful alternative to argatroban in the setting of heparin-induced thrombocytopenia. This article reviews the pharmacology and clinical studies of subcutaneous desirudin.
Thrombin is a serine protease generated in response to vascular injury and is an essential component of hemostatic clot formation. It catalyzes the conversion of soluble fibrinogen to fibrin, activates platelets, and amplifies its own generation by activating clotting factors V, VIII, and XI.1 Thrombin activity is regulated by endogenous anticoagulants such as antithrombin, which is a required cofactor for heparins (unfractionated heparin and low-molecular-weight heparin [LMWH]) to exert their anticoagulant effect.2 Thrombin concentration at the site of clot formation profoundly influences the structure of the fibrin fibers and the rigidity of the clot. High levels of thrombin result in clots composed of densely packed fibrin fibers that are relatively resistant to fibrinolysis.3 Therefore, thrombin is a major therapeutic target for the prevention or treatment of thromboembolism.
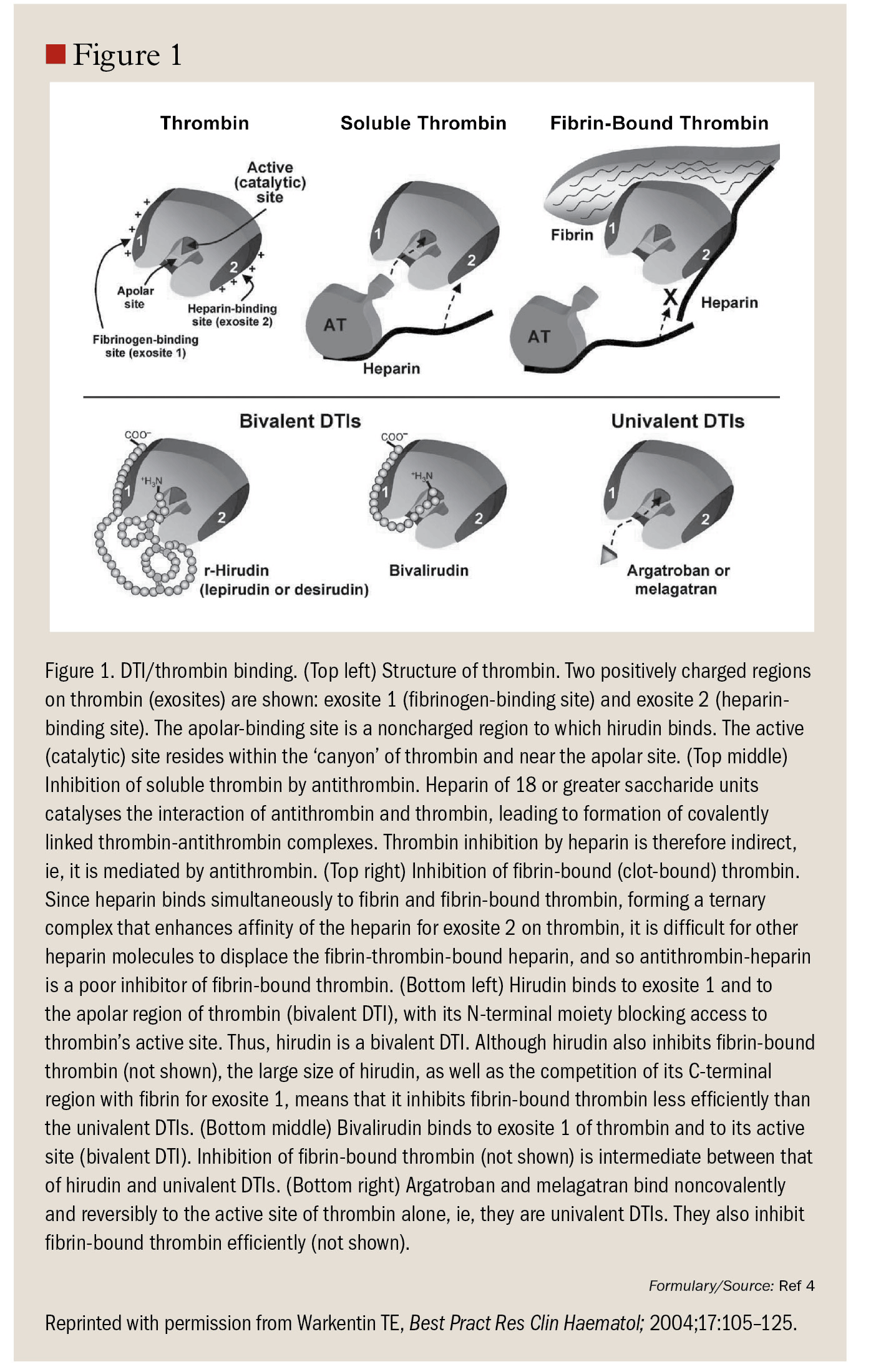
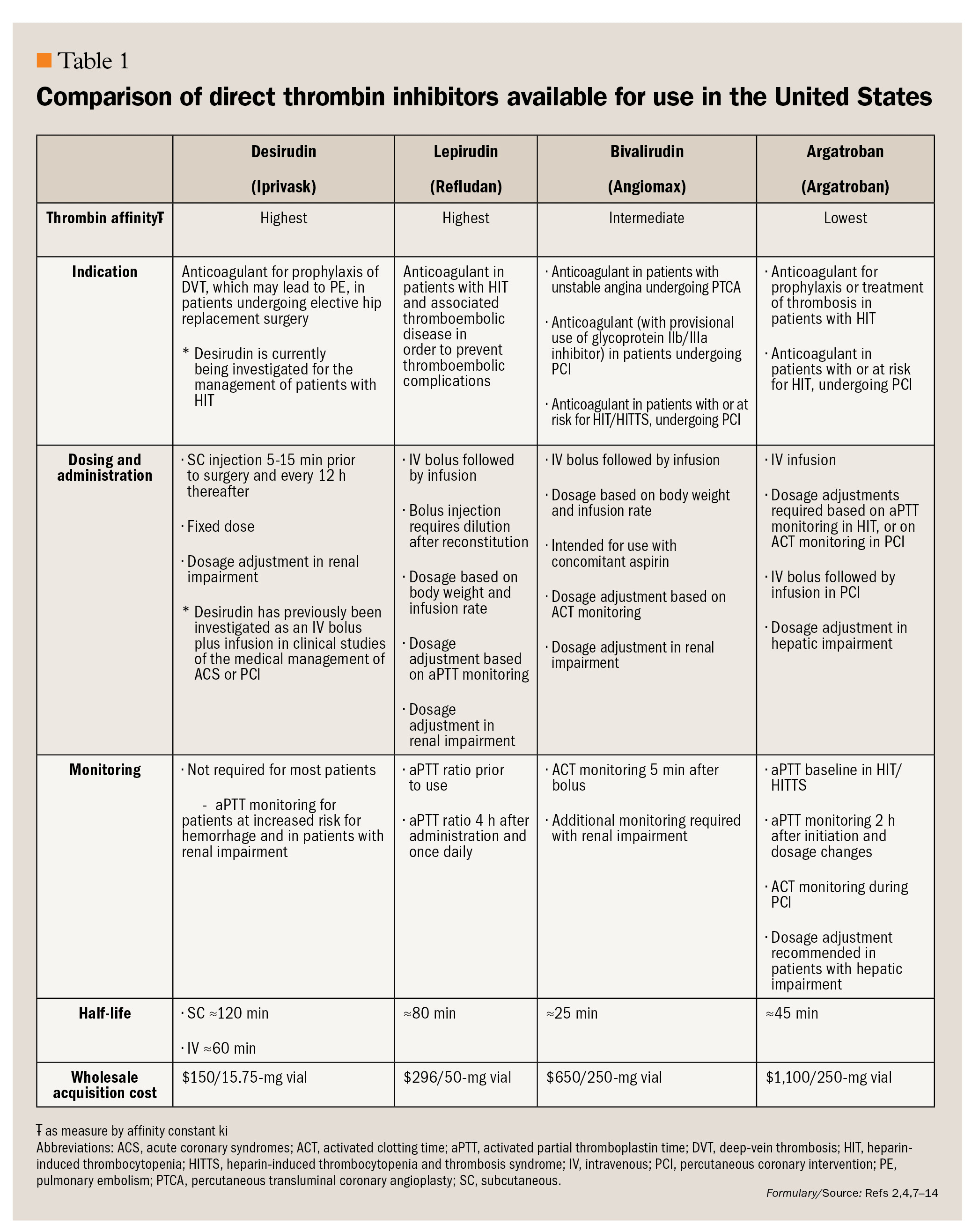
Direct thrombin inhibitors (DTIs) exert their anticoagulant effect by directly binding to thrombin molecules, and by inhibiting the thrombogenic activity of thrombin. DTI activity is not dependent on the presence of antithrombin.4 Unlike heparins, DTIs inhibit the activity of both circulating and clot-bound thrombin.5 DTIs have a low-binding affinity to plasma proteins and a more predictable dose response than unfractionated heparin.1 They are considered a mainstay of treatment for heparin-induced thrombocytopenia (HIT), an antibody-mediated syndrome that can lead paradoxically to life-threatening thrombosis.2,6Currently, 4 DTIs are approved for use as anticoagulants in the United States: desirudin (Iprivask, Canyon Pharmaceuticals), lepirudin (Refludan, Bayer HealthCare Pharmaceuticals), bivalirudin (Angiomax. The Medicines Company), and argatroban (Argatroban, GlaxoSmithKline).7-10 Each DTI has different indications and each has unique properties, including differences in potency, pharmacokinetics, monitoring requirements, and routes of administration (Table 1).2,4,7-14122139730 833Figure1.jpgFigure1.jpg In the United States, desirudin is currently indicated for the prophylaxis of deep-vein thrombosis (DVT), which may lead to pulmonary embolism (PE), in patients undergoing elective hip replacement surgery.9 It is the only DTI approved for subcutaneous (SC) administration at a fixed dose (15 mg every 12 hours).9 This article provides an overview of the pharmacology and clinical studies of subcutaneously administered desirudin.
PHARMACODYNAMICS
DTIs may be classified according to their binding interaction with thrombin (Figure 1). Bivalent DTIs (such as desirudin, lepirudin, and bivalirudin) block thrombin at 2 sites-the active (catalytic) site and exosite 1 (fibrinogen-binding site).1,4Univalent DTIs such as argatroban interact with thrombin only at the active site.1,4 It has been hypothesized that, because of their higher binding affinity for thrombin, bivalent DTIs may be more effective than univalent DTIs in preventing recurrent thrombotic events in patients with acute coronary syndromes.11 Desirudin is highly selective for thrombin over other serine proteases, such as coagulation factors and digestive enzymes.12 It has extremely high affinity for thrombin, with an affinity constant (ki) comparable to that of lepirudin and substantially higher than that of bivalirudin and argatroban (Table 1). 8,12-14
Unlike unfractionated heparin, desirudin appears to have minimal effects on platelet aggregation in healthy volunteers.15 Patients treated with a bolus intravenous (IV) injection of desirudin 1.0 mg/kg showed normal platelet aggregation induced by adenosine diphosphate, collagen, adrenaline, thrombofax, or ristocetin.15 Platelet counts remained in the normal range (>150,000/mm3) in several other studies of healthy volunteers after exposure to 0.1 to 1.0 mg/kg IV bolus doses of desirudin, infusions of 0.1 to 0.3 mg/kg/h, and consecutive SC injections at doses ranging from 0.3 to 0.5 mg every 8 hours for 3 days.16,17
The effects of desirudin on activated partial thromboplastin time (aPTT) have been evaluated after IV bolus (0.1–1.0 mg/kg) and SC (0.1–0.75 mg/kg) administration in healthy volunteers, after SC administration (10–20 mg twice daily) in patients undergoing hip replacement surgery, and after continuous IV infusion in patients with unstable angina (0.05–0.3 mg/kg/h for 72–120 hours).16-19 In general, these studies showed a clear correlation between increasing serum concentrations and prolongation of aPTT over a broad range of desirudin doses.16-19 After single SC doses in healthy volunteers, an effect on aPTT was observed within 30 minutes and was sustained at near maximal levels for 3 to 4 hours, returning to baseline within 24 hours.17 Prolongation of aPTT was maintained through 6 days of twice-daily SC administration in healthy volunteers and in patients undergoing hip replacement surgery, with no evidence of drug accumulation.18
PHARMACOKINETICS
Desirudin is rapidly absorbed after SC administration, reaching maximum drug plasma concentrations 1 to 3 hours after doses of 0.1 to 0.75 mg/kg, with nearly 100% bioavailability.17,20,21 Maximum drug concentrations (Cmax) and area under the drug concentration curve (AUC) are also proportional to the dose.17 Consistent pharmacokinetic parameters were observed in healthy volunteers given either single bolus IV injections of desirudin over a range of 0.1 to 1.0 mg/kg or continuous infusions over a range of 0.1 to 0.3 mg/kg/h.16,22 Distribution after IV dosing is rapid and is best described by a 2- or 3-compartment disposition model with first-order elimination kinetics.9,16,22-24 Plasma clearance following IV administration is also rapid, with 90% of the administered dose cleared within 2 hours, although prolongation of aPTT persists for about 1 hour following IV dosing versus about 2 hours following SC dosing.19,17,25
Desirudin is primarily metabolized and eliminated by the kidneys.2,21 Approximately 50% of the administered dose is eliminated unchanged in the urine.26,27 The disposition of the remainder of the drug is unknown.28 Minute amounts of the dose are eliminated as metabolites lacking 1 or 2 C-terminal amino acids, suggesting that desirudin undergoes stepwise degradation by carboxypeptidases in the kidney, with little or no hepatic metabolism.25 Desirudin has a mean terminal elimination half-life of approximately 2 to 3 hours following IV or SC administration.9,22
Two reports from investigations of the pharmacokinetics of desirudin in patients with renal impairment have been published. In the first study, the pharmacokinetics of single IV doses in subjects with mild, moderate, or severe renal impairment or normal renal function were compared.25 The mean dose-normalized AUC was shown to increase threefold in patients with moderate renal insufficiency (defined as creatinine clearance of 36-59 mL/min/1.73 m2) and sevenfold in those with severe renal insufficiency (defined as creatinine clearance of 12.1–27.2 mL/min/1.73 m2) compared with healthy volunteers.25 FDA-approved prescribing information for SC administration of desirudin in patients with renal impairment is based on the IV data from this single-dose study. According to the current prescribing information and the American College of Chest Physicians Practice Guidelines, the initial dose of desirudin should be reduced from the usual dose of 15 mg given subcutaneously every 12 hours to 5 mg every 12 hours in patients with moderate renal insufficiency (defined as creatinine clearance of 31–60 mL/min/1.73 m2) and to 1.7 mg given every 12 hours in patients with severe renal impairment (defined as creatinine clearance of 2).2,9 These current dosing recommendations are based on increases in total drug exposure (AUC) following single IV doses of desirudin. However, peak plasma concentration (Cmax) is a better predictor of maximal aPTT effect (and bleeding risk) than is AUC.28 It has been shown that the Cmax of desirudin is much lower following SC administration compared with a single IV dose.21,28
In a recent analysis of 6 studies that assessed the pharmacokinetics of desirudin in the setting of renal insufficiency, plasma concentration and aPTT were determined after administration of desirudin 15 mg SC given every 12 hours for up to 6 days in subjects with normal renal function and moderate renal impairment (creatinine clearance, 31–60 mL/min). Peak desirudin concentrations at steady state were 35 and 47 nmol/L in the normal and moderate renal impairment groups, respectively.28 There was only a 76% increase in exposure to desirudin over the dosing interval at steady state (AUC0-τ moderate renal impairment, 341 nmol•h/L; normal renal function, 194 nmol•h/L) in this analysis compared with a 300% increase observed in the single-dose, IV pharmacokinetic study.25,28 These recent pharmacokinetic data suggest that dose reduction of desirudin in patients with moderate renal impairment is not warranted and may result in subtherapeutic levels of desirudin.28
In a small study that included patients aged at least 65 years, mean plasma clearance of desirudin was decreased by 28% in the patients aged 65 years and older (n=12, 110 mL/min) compared with those who were younger than 65 years (n=8, 153 mL/min).9 However, a pharmacokinetic analysis of 301 patients undergoing hip replacement surgery showed that age and gender have no impact on systemic clearance of desirudin when renal function is taken into account.9 Desirudin has not been evaluated in patients with hepatic insufficiency, so dosing recommendations for this group cannot be made. Although there is no evidence that desirudin is metabolized in the liver, this patient population is at risk for coagulation abnormalities resulting from a reduction in vitamin K production in the liver. Therefore, caution is recommended when using desirudin in patients with impaired liver function. 9
DOSING, ADMINISTRATION, AND MONITORING
Unlike other DTIs currently available in the United States, desirudin can be administered subcutaneously at a fixed dose. The other DTIs require weight-based dose calculations and are administered via continuous infusion.7-10 The recommended dose of desirudin for DVT prophylaxis in adult patients undergoing elective hip replacement surgery is 15 mg every 12 hours administered as a SC injection. The initial dose should be administered 5 to 15 minutes prior to surgery but after induction of regional block anesthesia. As described previously, dose reductions are currently recommended in patients with moderate or severe renal impairment. The recently reported pharmacokinetic data are currently under review by FDA to determine if the prescribing information on dosing in patients with renal impairment should be modified. Desirudin is contraindicated in patients with a known hypersensitivity to natural or recombinant hirudins and in patients with active bleeding. In addition, as with other anticoagulants, desirudin should be used with caution in patients who also require concomitant therapy with antiplatelet agents, such as thienopyridines (eg, clopidogrel) and nonsteroidal anti-inflammatory drugs.9
Although patients receiving the indicated desirudin dosage do not require routine monitoring, it can easily be performed for patients who are at high risk for bleeding or for those with severe renal impairment.9,25,28 All thrombin-dependent coagulation assays are affected after administration of desirudin. Thrombin time values frequently exceed 200 seconds (values beyond the sensitivity limit of the test), which makes this test unsuitable for the monitoring of desirudin.17 The aPTT closely correlates with plasma levels of desirudin across a wide range of plasma concentrations following SC and IV administration.27,28 Thus, aPTT is the recommended assay for monitoring the anticoagulant effects of desirudin when desired.9,16,17,29 Daily monitoring of aPTT and serum creatinine is recommended in patients at high risk of bleeding or patients with severely impaired renal function. Current prescribing information recommends that if peak aPTT exceeds 2 times control, treatment with desirudin should be interrupted until aPTT falls to less than 2 times control.9 Treatment may then be reinitiated at a reduced dose.9
The effect of desirudin on international normalized ratio (INR) appears to be similar to the INR effect of lepirudin. Lepirudin increases INR by about 0% to 30%. This is less than that of argatroban, which increases INR by about 20% to 110%. The variations depend on the reagent used to measure the INR.30 In a study assessing the effect of desirudin on INR values in 12 healthy volunteers receiving concomitant warfarin, desirudin increased INR by about 10%.31 While desirudin does not routinely require coagulation monitoring, patient coagulation status (INR) should be monitored with all DTIs during transition to oral anticoagulant therapy.9 Monitoring is particularly important with argatroban, as its use may complicate the transition to oral anticoagulant therapy.2 Currently it is recommended that both heparin and nonheparin anticoagulants be overlapped with warfarin therapy, usually for a minimum of 5 days until the goal INR of 2.0 to 3.0 has been reached.6,9 The minimal effect on INR and the ability to administer desirudin via the SC route may facilitate the transition to warfarin therapy.
SAFETY AND TOLERABILITY
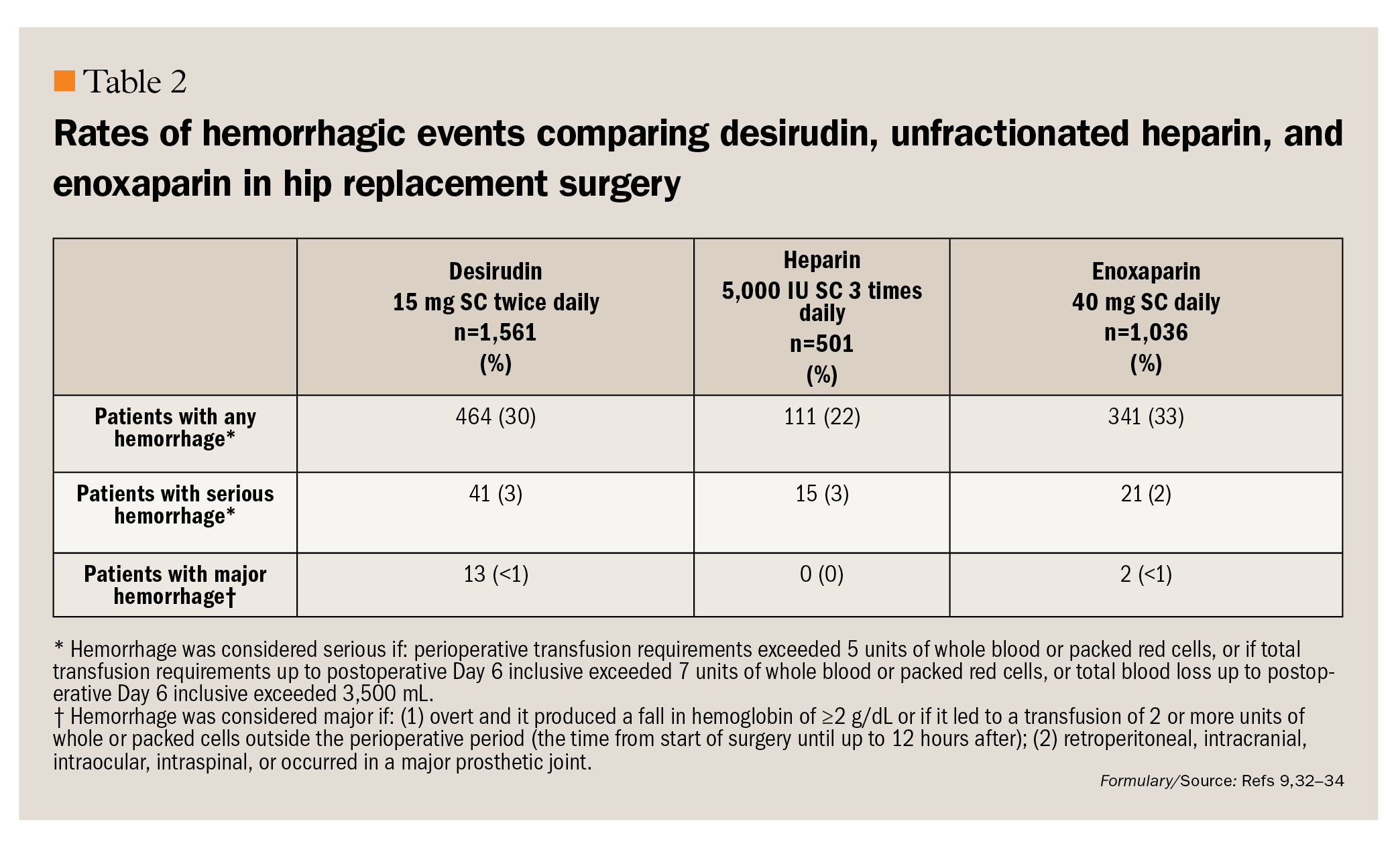
Bleeding. In 3 orthopedic studies assessing desirudin for venous thromboembolism (VTE) thromboprophylaxis, the safety and tolerability profile of twice-daily SC desirudin 15 mg was similar to that of thrice-daily SC low-dose unfractionated heparin (LDUH) 5,000 IU and once-daily SC enoxaparin 40 mg.32,34 Each of these studies used the currently approved dose and formulation of desirudin (15 mg SC twice daily) for a maximum duration of 11 to 12 days.33,34 Specifically, no differences in the incidence of bleeding events, including blood loss, transfusion requirements, and bleeding complications, were observed between treatment groups in these studies. The incidence of bleeding events in the combined desirudin, unfractionated heparin, and enoxaparin treatment groups is presented in Table 2.32,34 Of interest, in the study comparing desirudin and enoxaparin, the initial dose of enoxaparin was administered on the evening before surgery, whereas desirudin was given immediately prior to surgery.34 Despite the difference in timing of initial administration of the study medications, there were no differences between groups in safety-related outcomes, including blood loss, need for blood transfusion, and serious bleeding.34
Reversal of anticoagulant effects. There is no specific antidote to reverse the anticoagulant effects of DTIs (including desirudin) should significant bleeding arise; however, the half-life of desirudin is short (≈2 hours).2 While recombinant factor VIIa has been shown to reduce bleeding induced by DTIs in healthy volunteers, its clinical utility has not been established.2 In a prospective study of healthy volunteers, administration of desmopressin 0.3 μg/kg IV administered over 15 minutes reduced the anticoagulant (aPTT) effects of desirudin infused at doses of 0.2 and 0.3 mg/kg/h by about 25%, without affecting the plasma pharmacokinetics of desirudin.35 Desirudin can be cleared via hemodialysis with the use of special dialysis membranes.2
Allergenicity and immunogenicity. Desirudin appears to be a weak allergen. Allergic reactions have been reported in fewer than 2% of patients given desirudin in phase 3 clinical studies.9 Allergenicity was investigated in 263 healthy volunteers who were not previously exposed to the drug.36 Each participant had a first exposure to desirudin consisting of repeated SC doses over a 3- to 6-day period or a 6- or 72-hour IV infusion. Of these, 195 volunteers received single SC doses separated by at least 28 days for a second exposure, and 5 received single SC doses separated by at least 28 days for a third exposure. Four (1.5%) subjects experienced allergic reactions following administration of desirudin (3 occurred after IV administration; 1 occurred after SC administration); none were systemic reactions.36 No measurable effects on IgE levels and no formation of desirudin-specific IgG antibodies were detected in any of these participants through 56 days postexposure.36
However, no patients in this study were treated for longer than 6 days, which may be too short to induce an antihirudin antibody response.37 Greinacher and colleagues investigated immunization risk in 112 patients treated with SC desirudin 10, 15, or 20 mg administered twice daily for 8 to 12 days for the prevention of DVT following orthopedic surgery.38 Approximately 10% of patients developed IgG antihirudin antibodies by day 8.38 No differences in circulating desirudin levels or bleeding complications were observed between antidesirudin antibody positive and negative patients in this study.38 Based on these preliminary data, desirudin antibodies do not appear to affect desirudin activity.
Similar studies investigating the incidence of IgG antihirudin antibody formation in subjects exposed to lepirudin have reported much higher rates of antihirudin antibody formation (44%-74%), with approximately 27% of patients testing positive for antihirudin antibodies by day 8.37,39 The investigators concluded that antibody formation has a significant impact on the pharmacokinetics of lepirudin and may necessitate significant dose reductions.37 Bivalirudin does not appear to be immunogenic; however, antibodies to the other DTIs showed cross-reactivity with bivalirudin.2
CLINICAL EFFICACY OF DESIRUDIN: CURRENT AND POTENTIAL FUTURE INDICATIONS
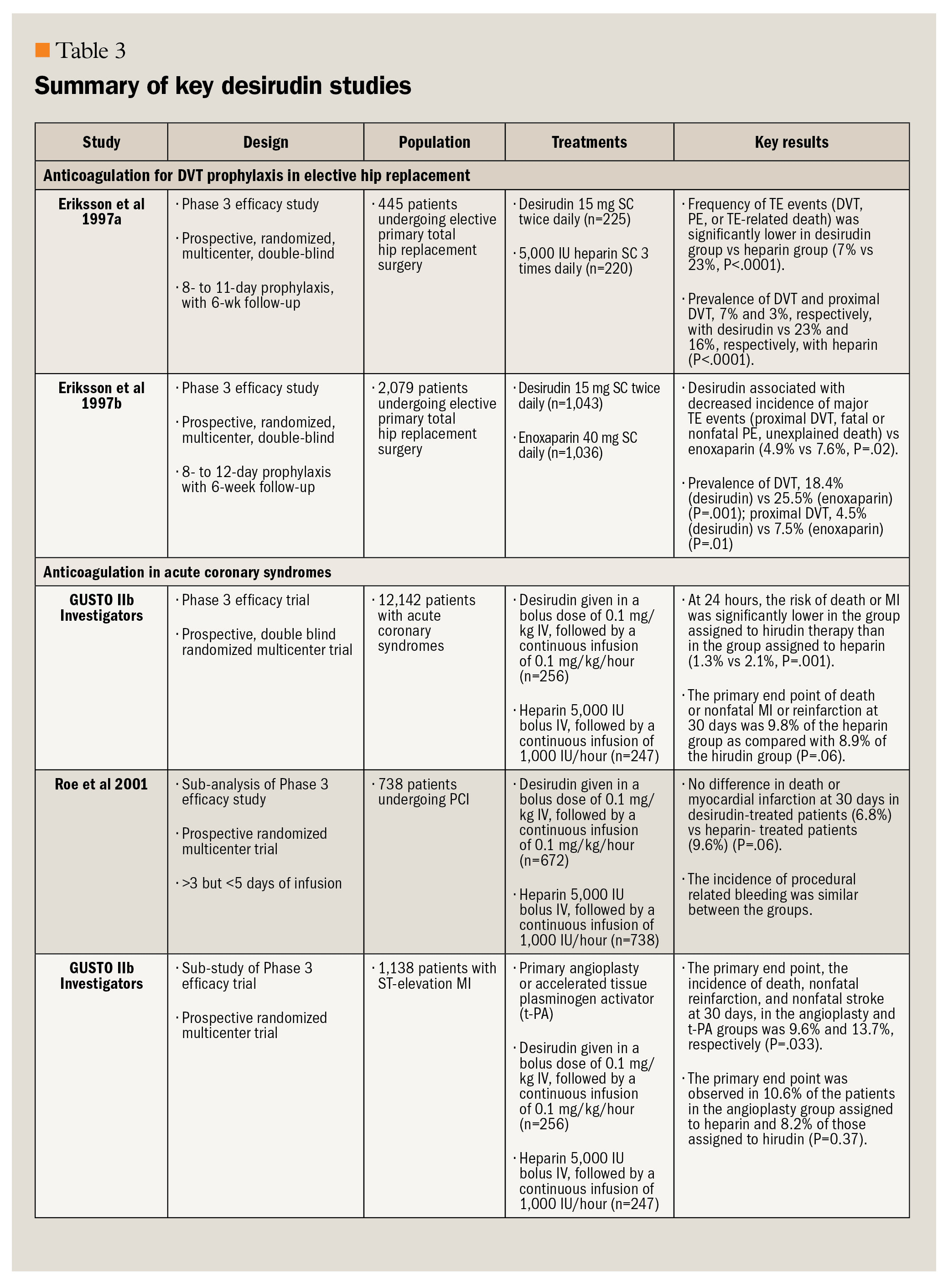
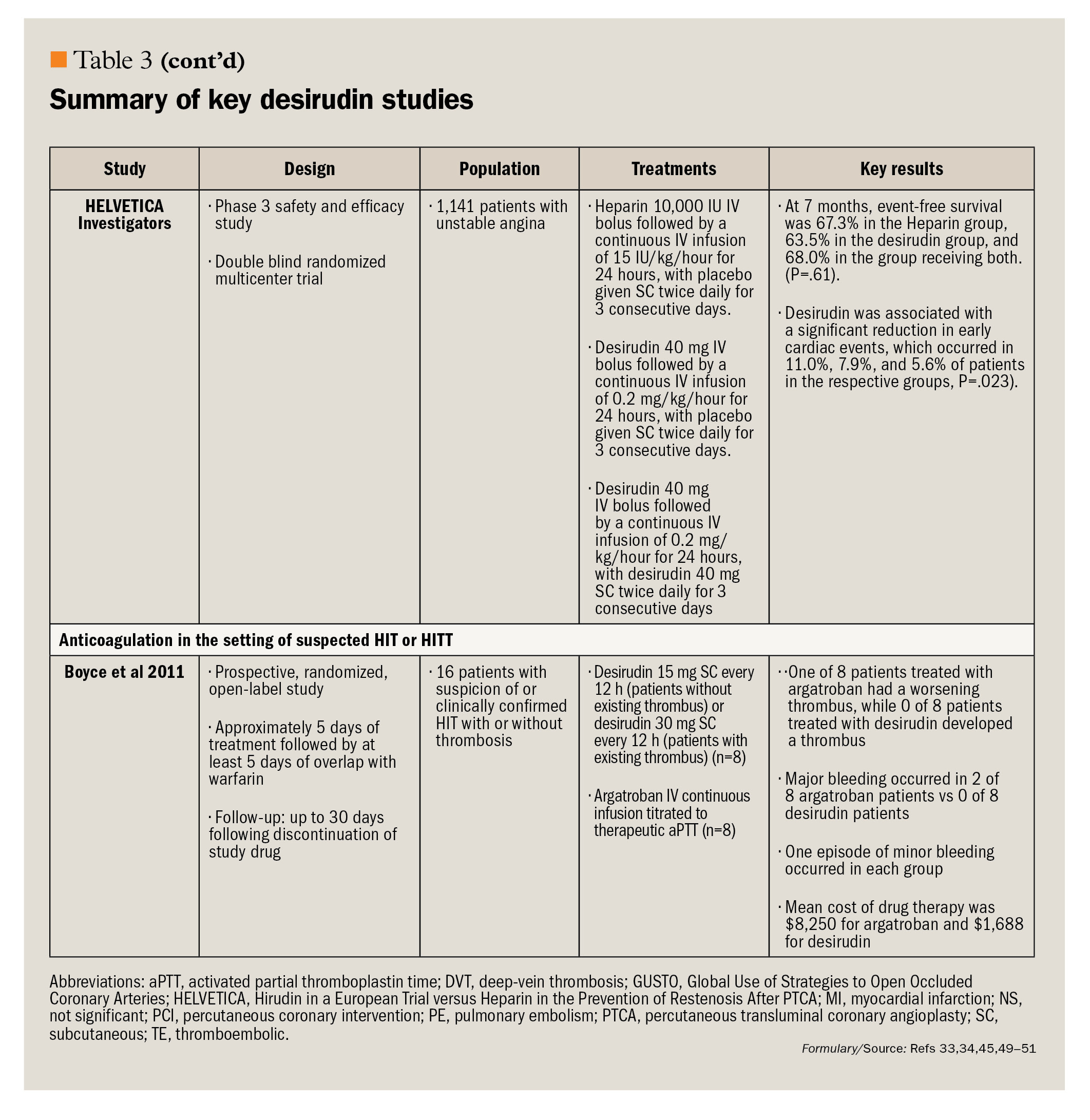
The clinical efficacy of desirudin in a high-risk surgical model-patients undergoing hip replacement surgery-has been demonstrated in 3 large, randomized, clinical studies.32-34 The use of desirudin as an anticoagulant in the medical management of acute coronary syndromes, in patients undergoing percutaneous coronary intervention, and in patients with suspected HIT has also been investigated.19,40-49 The most promising area for future use of desirudin appears to be in surgical and other patients at high risk for DVT (eg, cancer patients) and in patients with suspected HIT. A summary of the key desirudin studies in patients undergoing hip replacement surgery (current indication) as well as in patients with HIT (potential future indication) is presented in Table 3.33,34,46,49-51
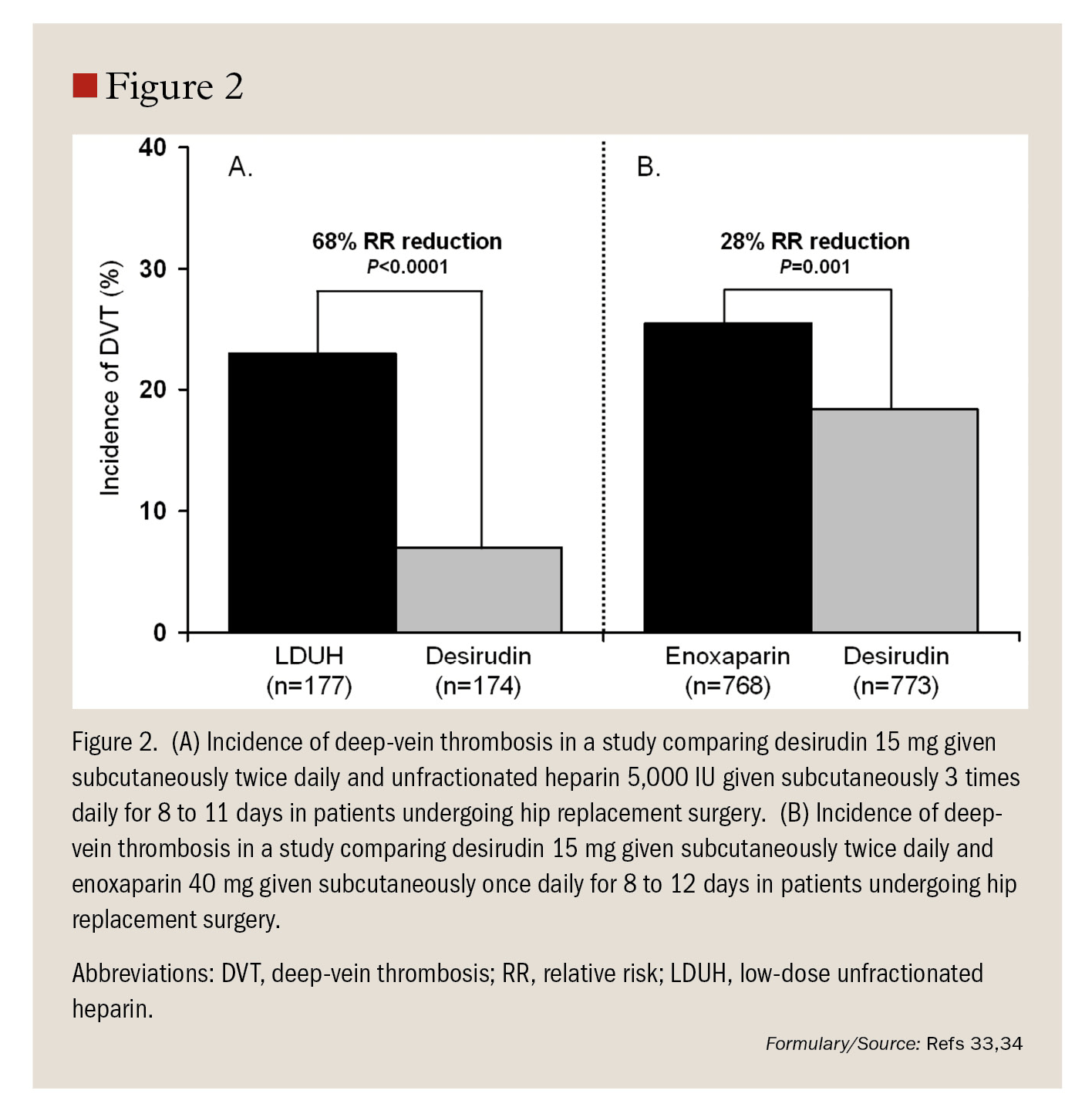
In 2 key studies assessing the efficacy of desirudin for prevention of thromboembolic events in patients undergoing elective hip replacement surgery, desirudin 15 mg SC administered twice daily for up to 12 days was superior to SC LDUH 5,000 IU given 3 times daily (n=445; incidence of thromboembolic events, desirudin 13/174 [7%] vs. LDUH 41/177 [23%]; PP=.02; Figure 2), with no significant differences in rates of bleeding complications among the treatment groups.33,34
A post hoc subgroup analysis of integrated data from the 2 key clinical studies described above and from an additional study comparing desirudin and LDUH in patients undergoing hip replacement surgery (n=2,307; 1,143 patients receiving desirudin) was conducted.32,52 In this analysis, patients were stratified according to age, type of anesthesia used during surgery, and the presence of obesity, cardiovascular disease, malignancy, or previous venous thromboembolic disease (VTD) at baseline. Incidence of VTE was then calculated by subgroup in patients treated with desirudin, LDUH, and enoxaparin. Venous thromboembolism outcomes were analyzed for each subgroup via multivariate logistic regression. Aged at least 65 years (VTE odd ratio, desirudin vs enoxaparin, 0.52; 95% CI, 0.40, 0.68; P=.001), obesity (0.69; 95% CI, 0.53, 0.90; P=.0054), history of VTD (0.58; 95% CI, 0.35, 0.96; P=.033), and malignancy interaction (0.12; 95% CI, 0.02, 0.63; P=.013) were associated with incremental therapeutic benefits in patients treated with desirudin compared with enoxaparin.52 In addition, there was a significant interaction effect between treatment and the number of patient risk factors. Patients with multiple risk factors who were treated with desirudin had fewer VTEs compared with those treated with enoxaparin or LDUH (P
Recent data suggest that desirudin may have potential utility as an anticoagulant in patients with clinically suspected HIT with or without thrombosis.49 PREVENT-HIT, an open-label, randomized, active control, exploratory study, assessed the clinical and economic utility of SC desirudin 15 or 30 mg administered twice daily compared with a continuous infusion of aPTT-adjusted argatroban for prevention of new or worsening thrombosis. The primary efficacy measure was the composite of new or worsening thrombosis that required discontinuation of study drug, amputation, or death from any cause. Other outcomes assessed included time to platelet count recovery, major and minor bleeding, and pharmacoeconomic parameters.
Sixteen patients were randomly assigned; 8 received desirudin and 8 were randomly assigned to argatroban. At study entry, 11 patients (70%) had positive heparin antibody tests, with similar frequency between treatment groups. One patient in the argatroban group had worsening of an existing thrombosis. However, the patient recovered and did not require discontinuation of study medication. There were no occurrences of thrombosis in the desirudin group, and no deaths or amputations occurred in either group. Two patients in the argatroban group experienced major bleeding; 1 patient experienced minor bleeding. No major bleeding occurred in the desirudin group; 1 patient experienced minor bleeding. Platelet count recovery was similar between the 2 groups.
PHARMACOECONOMIC EVALUATIONS
The economic utility of desirudin in VTE and thrombosis prevention or treatment in patients with HIT was recently investigated in 2 decision-tree models. Inputs for both of these models included drug costs, drug administration costs, daily patient hospitalization costs, antibody testing costs, and costs associated with adverse outcomes. All probabilities and inputs were either identified through a comprehensive literature search or through clinical input from experts and data on file. Patient characteristics were extracted from major clinical studies of VTE and HIT.52
The first model evaluated outcomes associated with the administration of desirudin versus enoxaparin in high-risk orthopedic surgery patients requiring VTE prophylaxis. Four outcomes were evaluated and included well (ie, no bleeding, DVT, or PE), bleeding, DVT, or PE. The analysis indicated that desirudin and enoxaparin were associated with similar treatment costs (total blended costs of $22,500 and $20,219 for desirudin and for enoxaparin, respectively). Desirudin was, however, not associated with a risk of progressing to HIT.52
The second model evaluated outcomes of early desirudin administration versus argatroban in surgical and medical patients with suspected HIT following the initial administration of LDUH or LMWH for VTE prophylaxis, with the goal of preventing complications of HIT. Outcomes assessed for the HIT model were well (ie, no bleeding, new thrombosis, amputation, or death), bleeding, new thrombosis, amputation, or death. Patients were stratified based on risk of developing thrombosis and were allocated to 4 treatment arms. In patients with suspected HIT, desirudin was associated with substantially reduced overall costs ($4,707 for desirudin vs $6,991 for argatroban) in both high- and low-risk patients. The primary drivers of cost savings for desirudin compared with argatroban were reduced drug costs, reduced rates of new thromboses, fewer inpatient days spent waiting for test results, decreased resource intensity, and outpatient warfarin transfer. Based on these outcomes, it was concluded that desirudin is cost-saving compared with argatroban for the treatment of suspected HIT.52
Pharmacoeconomic parameters were also assessed in the PREVENT-HIT study, which compared desirudin to argatroban in patients with suspected HIT. Using wholesale acquisition costs, the mean total cost of study medication per course of treatment was $1,750 for desirudin and $8,250 for argatroban.52 Also, on average, patients treated with desirudin had a shorter duration of hospitalization and required fewer dose adjustments compared with the argatroban group.52
CONCLUSIONS
Desirudin is a potent, highly selective DTI that exhibits predictable pharmacokinetics when administered as a fixed-dose, SC injection. It is more effective than LDUH and LMWH for DVT prophylaxis in patients undergoing hip replacement surgery, and is the only DTI approved for this use in the United States. Based on the results from the exploratory PREVENT-HIT study, desirudin shows promise as a potentially cost-effective alternative to argatroban in patients with suspected HIT with or without thrombosis. Further studies in the setting of HIT are warranted.
REFERENCES
- Di Nisio M, Middeldorp S, Büller HR. Direct thrombin inhibitors. N Engl J Med. 2005;353:1028–1040.
- Hirsh J, Bauer KA, Donati MB, Gould M, Samama MM, Weitz JI. Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133:141S–159S.
- Wolberg AS, Monroe DM, Roberts HR, Hoffman M. Elevated prothrombin results in clots with an altered fiber structure: a possible mechanism of the increased thrombotic risk. Blood. 2003;101:3008–3013.
- Warkentin TE. Bivalent direct thrombin inhibitors: hirudin and bivalirudin. Best Pract Res Clin Haematol. 2004;17:105–125.
- Weitz JI, Leslie B, Hudoba M. Thrombin binds to soluble fibrin degradation products where it is protected from inhibition by heparin-antithrombin but susceptible to inactivation by antithrombin-independent inhibitors. Circulation. 1998;97:544–552.
- Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133:340S–380S.
- Angiomax [package insert]. Parsippany, NJ: The Medicines Company; 2005.
- Argatroban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2009.
- Iprivask [package insert]. Hunt Valley, MD: Canyon Pharmaceuticals, Inc; 2010.
- Refludan [package insert]. Wayne, NJ: Bayer HealthCare Pharmaceuticals; 2006.
- Direct Thrombin Inhibitor Trialists’ Collaborative Group. Direct thrombin inhibitors in acute coronary syndromes: principal results of a meta-analysis based on individual patients' data. Lancet. 2002;359:294–302.
- Talbot M. Biology of recombinant hirudin (CGP 39393): a new prospect in the treatment of thrombosis. Semin Thromb Hemost. 1989;15:293–301.
- Maraganore JM, Bourdon P, Jablonski J, Ramachandran KL, Fenton JW. Design and characterization of hirulogs: a novel class of bivalent peptide inhibitors of thrombin. Biochemistry. 1990;29:7095–7101.
- Parry MA, Maraganore JM, Stone SR. Kinetic mechanism for the interaction of hirulog with thrombin. Biochemistry. 1994;33:14807–14814.
- Hoet B, Tornai I, Arnout J, Close P, Vermylen J, Verstraete M. Open study of intravenous recombinant hirudin (CGP 39393) on platelet function and coagulation in healthy volunteers. Drug Invest. 1994;7:127–133.
- Marbet GA, Verstraete M, Kienast J, et al. Clinical pharmacology of intravenously administered recombinant desulfatohirudin (CGP 39393) in healthy volunteers. J Cardiovasc Pharmacol. 1993;22:364–372.
- Verstraete M, Nurmohamed M, Kienast J, et al, on behalf of the European Hirudin in Thrombosis Group. Biologic effects of recombinant hirudin (CGP 39393) in human volunteers. J Am Coll Cardiol. 1993;22:1080–1088.
- Cofrancesco E, Cortellaro M, Leonardi P, Corradi A, Ravasi F, Bertocchi F. Markers of hemostatic system activation during thromboprophylaxis with recombinant hirudin in total hip replacement. Thromb Haemost. 1996;75:407–411.
- Topol EJ, Fuster V, Harrington RA, et al. Recombinant hirudin for unstable angina pectoris. A multicenter, randomized angiographic trial. Circulation. 1994;89:1557–1566.
- Markwardt F, Nowak G, Stürzebecher J, Vogel G. Clinico-pharmacological studies with recombinant hirudin. Thromb Res. 1988;52:393–400.
- Fischer KG. The role of recombinant hirudins in the management of thrombotic disorders. BioDrugs. 2004;18:235–265.
- Cardot JM, Lefèvre GY, Godbillon JA. Pharmacokinetics of rec-hirudin in healthy volunteers after intravenous administration. J Pharmacokinet Biopharm. 1994;22:147–156.
- Markwardt F, Nowak G, Stűrzebecher J, Griessbach U, Walsmann P, Vogel G. Pharmacokinetics and anticoagulant effect of hirudin in man. Thromb Haemost. 1984;52:160–163.
- Markwardt F, Fink G, Kaiser B, et al. Pharmacological survey of recombinant hirudin. Pharmazie. 1988;43:202–207.
- Lefèvre G, Duval M, Gauron S, et al. Effect of renal impairment on the pharmacokinetics and pharmacodynamics of desirudin. Clin Pharmacol Ther. 1997;62:50–59.
- Meyer BH, Luus HG, Műller FO, Badenhorst PN, Röthig HJ. The pharmacology of recombinant hirudin, a new anticoagulant. S Afr Med J. 1990;78:268–278.
- Bichler J, Fichtl B, Siebeck M, Fritz H. Pharmacokinetics and pharmacodynamics of hirudin in man after single subcutaneous and intravenous bolus administration. Arzneimittelforschung. 1988;38:704–710.
- Nafziger AN, Bertino JS Jr. Desirudin dosing and monitoring in moderate renal impairment. J Clin Pharmacol. 2010;50:614–622.
- Talbot MD, Ambler J, Butler KD, et al. Recombinant desulphatohirudin (CGP 39393) anticoagulant and antithrombotic properties in vivo. Thromb Haemost. 1989;61:77–80.
- Gosselin RC, Dager WE, King JH, et al. Effect of direct thrombin inhibitors, bivalirudin, lepirudin, and argatroban, on prothrombin time and INR values. Am J Clin Pathol. 2004;121:593–599.
- Levy J, Kurz M, Whelton A. Lack of clinically significant interactions between the subcutaneously administered direct thrombin inhibitor desirudin and orally administered warfarin upon the international normalized ratio. Presented at: the 51st American Society of Hematology Annual Meeting and Exposition; December 5-8, 2009; New Orleans, LA. Abstract 22416.
- Eriksson BI, Ekman S, Kalebo P, Zachrisson B, Bach D, Close P. Prevention of deep-vein thrombosis after total hip replacement: direct thrombin inhibition with recombinant hirudin, CGP 39393. Lancet. 1996;347:635–639.
- Eriksson BI, Ekman S, Lindbratt S, et al. Prevention of thromboembolism with use of recombinant hirudin. Results of a double-blind, multicenter trial comparing the efficacy of desirudin (Revasc) with that of unfractionated heparin in patients having a total hip replacement. J Bone Joint Surg Am. 1997;79:326–333.
- Eriksson BI, Wille-Jorgensen P, Kalebo P, et al. A comparison of recombinant hirudin with a low-molecular-weight heparin to prevent thromboembolic complications after total hip replacement. N Engl J Med. 1997;337:1329–1335.
- Amin DM, Mant TG, Walker SM, et al. Effect of a 15-minute infusion of DDAVP on the pharmacokinetics and pharmacodynamics of REVASC during a four-hour intravenous infusion in healthy male volunteers. Thromb Haemost. 1997;77:127–132.
- Close P, Bichler J, Kerry R, et al. Weak allergenicity of recombinant hirudin CGP 39393 (REVASC) in immunocompetent volunteers. The European Hirudin in Thrombosis Group (HIT Group). Coron Artery Dis. 1994;5:943–949.
- Eichler P, Friesen HJ, Lubenow N, Jaeger B, Greinacher A. Antihirudin antibodies in patients with heparin-induced thrombocytopenia treated with lepirudin: incidence, effects on aPTT, and clinical relevance. Blood. 2000;96:2373–2378.
- Greinacher A, Eichler P, Albrecht D, Strobel U, Potzsch B, Eriksson BI. Antihirudin antibodies following low-dose subcutaneous treatment with desirudin for thrombosis prophylaxis after hip-replacement surgery: incidence and clinical relevance. Blood. 2003;101:2617–2619.
- Huhle G, Hoffmann U, Song X, Wang LC, Heene DL, Harenberg J. Immunologic response to recombinant hirudin in HIT type II patients during long-term treatment. Br J Haematol. 1999;106:195–201.
- The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) IIa Investigators. Randomized trial of intravenous heparin versus recombinant hirudin for acute coronary syndromes. Circulation. 1994;90:1631–1637.
- Cannon CP, McCabe CH, Henry TD, et al; for the TIMI 5 Investigators. A pilot trial of recombinant desulfatohirudin compared with heparin in conjunction with tissue-type plasminogen activator and aspirin for acute myocardial infarction: results of the Thrombolysis in Myocardial Infarction (TIMI) 5 trial. J Am Coll Cardiol. 1994;23:993–1003.
- Antman EM; for the TIMI 9A Investigators. Hirudin in acute myocardial infarction. Safety report from the Thrombolysis and Thrombin Inhibition in Myocardial Infarction (TIMI) 9A trial. Circulation. 1994;90:1624–1630.
- Lee LV; for the TIMI 6 Investigators. Initial experience with hirudin and streptokinase in acute myocardial infarction: results of the Thrombolysis in Myocardial Infarction (TIMI) 6 trial. Am J Cardiol. 1995;75:7–13.
- Antman EM; for the TIMI 9B Investigators. Hirudin in acute myocardial infarction. Thrombolysis and Thrombin Inhibition in Myocardial Infarction (TIMI) 9B trial. Circulation. 1996;94:911–921.
- The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) IIb Investigators. A comparison of recombinant hirudin with heparin for the treatment of acute coronary syndromes. N Engl J Med. 1996;335:775–782.
- Roe MT, Granger CB, Puma JA, et al. Comparison of benefits and complications of hirudin versus heparin for patients with acute coronary syndromes undergoing early percutaneous coronary intervention. Am J Cardiol. 2001;88:1403–1406.
- Serruys PW, Herrman JP, Simon R, et al; for the Helvetica Investigators. A comparison of hirudin with heparin in the prevention of restenosis after coronary angioplasty. N Engl J Med. 1995;333:757–763.
- van den Bos AA, Deckers JW, Heyndrickx GR, et al. Safety and efficacy of recombinant hirudin (CGP 39 393) versus heparin in patients with stable angina undergoing coronary angioplasty. Circulation. 1993;88:2058–2066.
- Boyce SW, Bandyk DF, Bartholomew JR, Frame JN, Rice L. A randomized, open-label pilot study comparing desirudin and argatroban in patients with suspected heparin-induced thrombocytopenia with or without thrombosis: PREVENT-HIT study. Am J Ther. 2011;18:14–22.
- Serruys P, Deckers JW, Close P; on behalf of the HELVETICA study group. A double-blind, randomized, heparin-controlled trial evaluating acute and long term efficacy of recombinant hirudin (CGP 39393) in patients undergoing coronary angioplasty. Circulation. 1994;90:a–2115.
- The Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes (GUSTO IIb) Angioplasty Substudy Investigators. A clinical trial comparing primary coronary angioplasty with tissue plasminogen activator for acute myocardial infarction. N Engl J Med. 1997;336:1621–1628.
- Data on file. Hunt Valley, MD: Canyon Pharmaceuticals Inc; 2010.
FDA Issues Complete Response Letter for Pz-Cel to Treat Epidermolysis Bullosa
April 22nd 2024Prademagene zamikeracel is a cell therapy designed to incorporate the functional collagen-producing COL7A1 gene into a patient’s own skin cells. The FDA is asking for additional information on manufacturing practices.
