- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
The current state of HIV therapy [INFOGRAPHIC]
Incidence of human immunodeficiency virus (HIV) has decreased dramatically since its emergence in the early 1980s, but it remains a worldwide epidemic. There is a reduction in newly diagnosed patients, but prevalence is increasing due to a longer life expectancy, which is attributed in part to highly effective antiretroviral therapies. Newly approved and investigational antiretroviral therapies provide additional options for the healthcare team to prevent progression of disease as well as transmission of HIV. Early detection and prevention of HIV is still paramount with the use of in-home HIV testing as well as antiretrovirals for pre-exposure prophylaxis. While many advances in HIV diagnosis and treatment have been made, the importance of education and risk avoidance cannot be underestimated.
ABSTRACT
Incidence of human immunodeficiency virus (HIV) has decreased dramatically since its emergence in the early 1980s, but it remains a worldwide epidemic. There is a reduction in newly diagnosed patients, but prevalence is increasing due to a longer life expectancy, which is attributed in part to highly effective antiretroviral therapies. Newly approved and investigational antiretroviral therapies provide additional options for the healthcare team to prevent progression of disease as well as transmission of HIV. Early detection and prevention of HIV is still paramount with the use of in-home HIV testing as well as antiretrovirals for pre-exposure prophylaxis. While many advances in HIV diagnosis and treatment have been made, the importance of education and risk avoidance cannot be underestimated.
The number of people newly infected with HIV has declined, but it remains a worldwide epidemic. Prevalence continues to rise with current estimates of 34 million people living with HIV or acquired immunodeficiency syndrome (AIDS), up from 29.4 million in 2001, due to new infections as well as patients living longer. Worldwide in 2011, 1.7 million people died of AIDS.1 In the United States the prevalence is estimated to be 1.2 million, with approximately 1 in 5 people infected who are undiagnosed and unaware.2 Although the HIV mortality rate has decreased 80% since its peak in 1995, HIV was the sixth leading cause of death for those aged 25 to 44 in 2009.3 Deaths have declined in part due to increased treatment options and utilization of highly effective antiretroviral medications.
Eradication of HIV is currently not feasible due to latent infection of CD4 cells; therefore, the goals of therapy include reducing morbidity and prolonging life, restoring and preserving immune function, decreasing opportunistic infections, decreasing viral load, limiting adverse events (AEs), and preventing further transmission. Preventing the emergence of resistance is also imperative. This can be best accomplished by achieving maximal and durable suppression of plasma viremia, which often requires the use of preferably 3 antiretroviral drugs (ARVs). The increasing availability of ARVs and development of new drug classes makes this a more feasible goal for all patients. Predictors of success include a rapid reduction in viral load, increased potency of the regimen, low baseline viremia, higher baseline CD4 count, and adherence to medication regimens.4 It has been estimated that in order to maintain viral load suppression, high medication adherence rates of greater than 95% may be required. Poor virologic and immunologic responses to ARVs lead to the development of drug-resistant virus.5
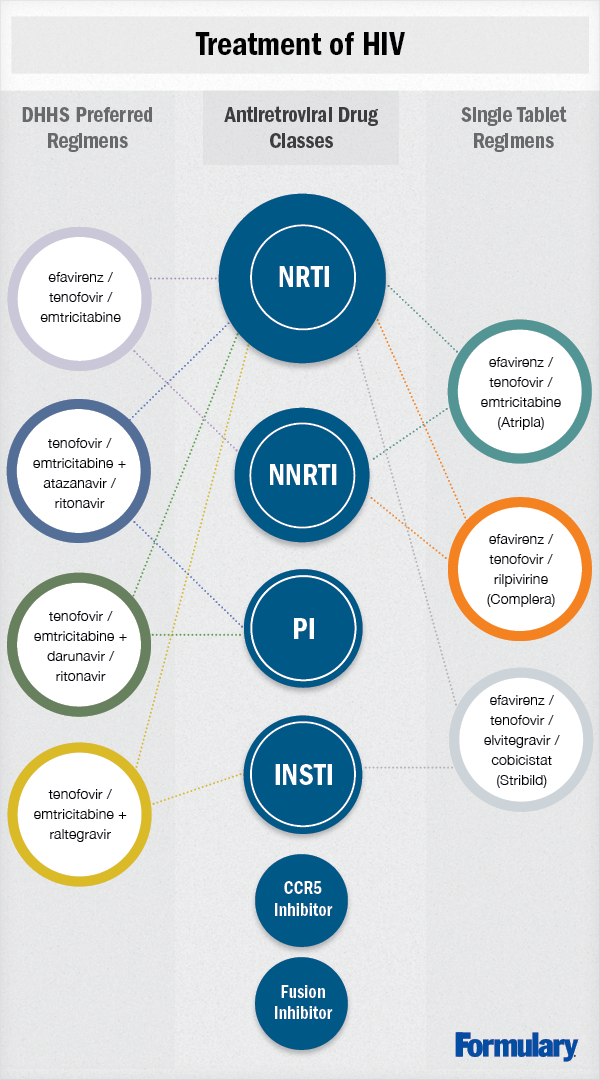
INITIATION OF TREATMENT
In February 2013, the Department of Health and Human Services (DHHS) Panel on Antiretroviral Guidelines for Adults and Adolescents provided an update to the Guidelines for the Use of Antiretroviral Agents in HIV-1−Infected Adults and Adolescents with recommendations on the initiation of antiretroviral therapy (ART) in treatment-naive patients. Recommended changes were made to reflect emerging data outlining the benefit of therapy in reducing transmission and the harmful effects of ongoing HIV replication on disease progression. These recommendations and their associated strengths are shown in Table 1.4
When deciding to initiate treatment, patients need to be educated on the importance of adherence along with the benefits and risks associated with ART (strength of recommendation: AIII; see Table 1). Evidence has shown that untreated HIV infection can lead to the development of other diseases including cardiovascular disease, kidney disease, liver disease, neurologic complications, and malignancies.4 Patients may choose to postpone therapy, and providers may elect to defer therapy on the basis of clinical or psychosocial factors such as lack of prescription drug coverage, depression, literacy level, and ability and willingness to initiate therapy and follow medication regimens.4
Antiretroviral therapy should consist of 2 nucleoside reverse transcriptase inhibitors (NRTIs) and at least 1 ARV from another class, including a non-nucleoside reverse transcriptase inhibitor (NNRTI), integrase strand transfer inhibitor (INSTI), or protease inhibitor (PI) for initial therapy. The Panel recommends the following as preferred regimens for treatment-naive patients.4 Their strengths of recommendation follow in parentheses.
• efavirenz/tenofovir disoproxil fumarate/emtricitabine (AI)
• ritonavir-boosted atazanavir + tenofovir disoproxil fumarate/emtricitabine (AI)
• ritonavir-boosted darunavir + tenofovir disoproxil fumarate/emtricitabine (AI)
• raltegravir + tenofovir disoproxil fumarate/emtricitabine (AI)
The selection of a regimen should be individualized on the basis of virologic efficacy, toxicity, pill burden, dosing frequency, drug-drug interaction potential, resistance testing results, and comorbid conditions. Recommended alternative and other regimens are also included in the guidelines.4
Antiretroviral agents and Current Targets of Therapy
ARVs target specific processes necessary for the replication of the HIV virus in the human host (Figure). Upon entry in the body the virus has contained within its outer shell or capsid all of the necessary elements, such as viral ribonucleic acid (RNA), for replication. The outer layer of the capsid is a protein receptor (gp120) that has affinity for CD4 receptors. These CD4 receptors, found on a number of cells in the body, have particular importance on CD4 T lymphocytes. An interaction between the gp120 receptor and the co-receptors CXCR4 and CCR5 on the CD4 cell reveals the gp41, thus allowing the fusion of the viral envelope to the CD4 plasma membrane. Once in the cytoplasm, viral reverse transcriptase transcribes the RNA into deoxyribonucleic acid (DNA) for transport into the cell nucleus and integration into the host cell’s genome. After HIV DNA is integrated into the host cell’s DNA, replication is induced. Once the lymphocyte is activated transcription of the viral DNA occurs, resulting in multiple copies of viral RNA. This RNA then codes or translates for the viral proteins and enzymes. An HIV enzyme protease cuts the long chains of HIV proteins into smaller individual proteins. As the smaller HIV proteins assemble with copies of viral RNA genetic material, a new virus particle is formed. These immature virions then mature to become active.6
Nucleoside reverse transcriptase inhibitors (NRTIs)
The NRTIs work by competing with nucleosides for incorporation into the viral DNA, thereby inhibiting the activity of HIV reverse transcriptase. This leads to chain termination and prevents replication.6 Currently available agents include abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, and zidovudine. A lack of CYP metabolism leads to fewer drug interactions; however, due to renal clearance, dose adjustments are required for renal insufficiency. All NRTIs may be administered without regard to food with the exception of didanosine.4 When given alone, it requires administration on an empty stomach; however, when given with tenofovir, this food restriction is eliminated.7 Class side effects include lactic acidosis and hepatomegaly.4 It should be noted that although the package inserts list these as class effects, they generally occur in very low incidence with certain agents (abacavir, emtricitabine, lamivudine, tenofovir). Some NRTIs also cause peripheral neuropathy (didanosine, stavudine4), and are thus not commonly used in practice.
Non-nucleoside reverse transcriptase inhibitors (NNRTIs)
The NNRTIs bind directly to reverse transcriptase and impede the RNA-dependent and DNA-dependent DNA polymerase activities.6 Currently available agents include delavirdine, efavirenz, etravirine, nevirapine, and rilpivirine. Class effects include rash and hepatotoxicity. A small percentage of patients experience a severe rash, including Stevens-Johnson syndrome. Nevirapine in particular has been associated with hepatotoxicity, especially in patients with higher CD4 counts, and is contraindicated in females with CD4 counts >250 cells/mm3 and males with CD4 counts >400 cells/mm3.8 Efavirenz and rilpivirine have been associated with neurologic and psychiatric AEs.. A recent study looking at the results of the ECHO and THRIVE trials showed that rilpivirine was associated with fewer neurological and psychiatric AEs than efavirenz over 48 weeks in treatment-naive, HIV-1−infected adults.9 The NNRTIs are all extensively metabolized by CYP3A and, with the exception of rilpivirine, are either CYP3A inhibitors or inducers. Therefore, as the potential for drug interactions is high, caution should be used when these agents are used in conjunction with potent CYP inhibitors, inducers, or substrates. It is vital to refer to package inserts or other reference materials when initiating or changing therapy. There is no food restriction with nevirapine administration. Efavirenz should be administered on an empty stomach.to reduce potential side effects, and it is recommended to administer rilpivirine and etravirine with a meal.4
Protease inhibitors (PIs)
These PIs inhibit HIV protease used to cleave proteins for final assembly of new virions, which subsequently induces the formation of immature noninfectious viral particles.6 The currently available agents include atazanavir, darunavir, fosamprenavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, and tipranavir. Side effects include gastrointestinal upset, lipodystrophy, dyslipidemia, hyperglycemia, and hepatotoxicity. As all PIs are substrates and inhibitors of CYP enzymes, specifically CYP3A, caution is advised if coadministered with medications known to be substrates, inhibitors, or inducers of these CYP enzymes. Most PIs should be administered with food (atazanavir, darunavir, nelfinavir, ritonavir, saquinavir). Both fosamprenavir and tipranavir should be administered with food if given with ritonavir tablets.4
CCR5 inhibitors
The CCR5 co-receptor antagonist inhibits fusion of HIV with the host cell by inhibiting the interaction between the gp120 viral glycoprotein and the CCR5 receptor.10 Maraviroc is currently the only available agent in this class. It is indicated for patients who carry CCR5 tropic virus; therefore, testing for co-receptor tropism is required prior to initiation. The co-receptor tropism assay reports the result as R5 (virus that utilizes the CCR5 receptor), X4 (virus that utilizes the alternate receptor CXCR4), or dual/mixed virus (which utilizes either or both receptors to enter the CD4 cell). The most commonly reported side effects include dizziness and muscle pain.4 Although a single case of hepatotoxicity with allergic features was reported,11, a cross-protocol analysis revealed no significant difference in hepatic toxicity between maraviroc and control groups.12 Concerns about CCR5 antagonists and malignancy were also addressed in a cross-protocol analysis, which showed no association.13 Maraviroc is metabolized by CYP3A, and the dose must be adjusted if given with CYP3A inhibitors or inducers. Maraviroc may be administered without regard to meals.11
Fusion inhibitors
The fusion inhibitor functions by inhibiting fusion of viral and cellular membranes and thus entry into the CD4 cell. It binds to the first heptad-repeat (HR1) in the gp41 subunit of the viral envelope glycoprotein and prevents conformational changes required for fusion of the viral and cellular membranes. Enfuvirtide is the only currently available fusion inhibitor and is available as a subcutaneous injection. The most commonly reported AEs are injection-site reactions including redness, swelling, pain, hardened skin, and bumps. There are no clinically relevant drug interactions with enfuvirtide, and due to subcutaneous administration it can be administered without regard to meals.14 Currently in the management of HIV, this agent is generally reserved for salvage therapy due to its side effect profile and the availability of newer highly effective oral agents.15
Integrase strand transfer inhibitors (INSTIs)
INSTIs obstruct integrase by binding in the catalytic core domain of the enzyme and competing for binding with the host DNA. This prevents integrase from inserting the viral genome into the host DNA. The currently available individual agent is raltegravir, with common AEs of insomnia, headache, nausea, and fatigue. Raltegravir is mainly metabolized via a UDP-glucuronosyltransferases (UGT) 1A1-mediated glucuronidation pathway. Decreased plasma concentrations may occur if coadministered with drugs that strongly induce UGT1A1 such as rifampin.16 With recent reports of myopathy associated with raltegravir, it should be used with caution in individuals with increased risk of myopathy or rhabdomyolysis17, such as those receiving concomitant therapy with statins and fibric acid derivatives. Raltegravir may be administered without regard to meals.16
A new INSTI, elvitegravir, is currently only available as combination with emtricitabine, tenofovir, and cobicistat, known as Stribild™. It requires a pharmacokinetic booster, cobicistat, for viral activity.18
NEWER THERAPIES
Recent approvals of ARVs have focused on single-tablet regimens. Prior to this, efavirenz/emtricitabine/tenofovir (Atripla®) was the only medication that was composed of 3 ARVs that could be taken as 1 tablet once daily. In May 2011, FDA approved rilpivirine, and in August 2011, the second single-tablet regimen was approved as emtricitabine/rilpivirine/tenofovir (Complera®). This was joined by elvitegravir/cobicistat/emtricitabine/tenofovir (Stribild™) in August 2012 as the third single-tablet regimen.19
Rilpivirine
Rilpivirine a newer NNRTI, has the advantage of having a smaller pill size compared to other ARVs. In the ECHO and THRIVE trials, rilpivirine was shown to have noninferior efficacy compared to efavirenz, with a higher virologic failure rate but a more favorable safety and tolerability profile in treatment-naive patients.20 A distinct advantage for rilpivirine is the lower rate of CNS side effects that are commonly seen with efavirenz.9 In these studies, patients with a baseline HIV-1 RNA >100,000 c/mL and CD4 counts <200 cells/mm3 experienced higher virologic failure rates20 prompting label indication of use in those with HIV-1 RNA <100,000 c/mL.21 Rilpivirine primarily undergoes oxidative metabolism mediated by the CYP3A system; hence, monitoring for drug interactions are important. Its exposure is approximately 40% lower when taken in a fasted condition compared with a normal caloric meal (533 kcal).21 Rilpivirine was shown in the ECHO and THRIVE trials to be better tolerated than efavirenz, but 10% of patients treated with rilpivirine experienced treatment failure, which were mostly virologic failure. The E138K mutation was the most common and affects susceptibility to all other available NNRTIs.23 Coadministration of rilpivirine with medications that elevate gastric pH such as proton pump inhibitors, H2 antagonists, and antacids may decrease the serum concentration of rilpivirine, resulting in potential virologic failure and possible resistance. Rilpivirine must be taken 4 hours before or 2 hours after antacids as well as 4 hours before or 12 hours after an H2 receptor antagonist. The use of rilpivirine with proton pump inhibitors is contraindicated.21 Providers must weigh these considerations carefully with each patient individually.
The combination of emtricitabine/rilpivirine/tenofovir, as CompleraÒ, is another single-tablet regimen option. Renal dose adjustment is not required; however the combination should not be used in patients with creatinine clearance (CrCl) <50mL/min. It is recommended that this ARV be taken with a meal. Again, as this agent includes rilpivirine, caution is recommended with the use of concomitant strong CYP inhibitors and acid-reducing agents.22
Elvitegravir/cobicistat/emtricitabine/tenofovir (Stribild™)
As the most recently approved INSTI-based single-tablet regimen of elvitegravir/cobicistat/emtricitabine/tenofovir for treatment-naive patients, elvitegravir utilizes cobicistat, a strong inhibitor of CYP3A, as a pharmacokinetic boosting agent. Caution is warranted with concomitant medications that are substrates, inducers, or inhibitors of CYP3A as both elvitegravir and cobicistat are metabolized by CYP3A, and cobicistat is metabolized to a minor extent by CYP2D6. This ARV is not recommended to be initiated in patients with CrCl <70 mL/min and should be discontinued when CrCl <50 mL/min. Generally this elevation in serum creatinine occurs within the first few weeks of therapy, which then stabilizes. There is a boxed warning of lactic acidosis and severe hepatomegaly with steatosis and post-treatment exacerbation of hepatitis B in patients who have discontinued emtricitabine or tenofovir. The most common side effects in clinical trials were nausea (16%) and diarrhea (12%). No dosage adjustment or changes of administration times are necessary if taken with proton pump inhibitors or H2 antagonists; however, it is recommended to separate Stribild™ and antacids by at least 2 hours. Patients should take the medication with food to enhance absorption.23 In September 2012, the DHHS Panel made the recommendation of use of the product as an alternative regimen for ART-naive HIV-infected patients with CrCl >70 mL/min (strength of recommendation: BI).4 Currently, neither elvitegravir nor cobicistat are available as separate agents.
ANTIRETROVIRALS on the Horizon
Dolutegravir
Dolutegravir is a once daily unboosted INSTI, distinctive from the currently licensed raltegravir and elvitegraivr, by way of low variability in its pharmacokinetics and a predicable dose-response relationship. It has been shown to have a high genetic barrier, exhibiting a different resistance profile and activity against isolates resistant to current INSTIs. Dolutegravir has shown in vitro activity against HIV-1 and HIV-2, which was independent of HIV subtype. Mainly metabolized by glucuronidation utilizing UGT1A1, with a minor role of CYP3A4, potential drug interactions may occur. It has a terminal elimination half-life of 13 to 15 hours, supporting the once daily dosing, and food administration does not alter drug exposure.24 Dolutegravir has been shown to inhibit renal tubular secretion of creatinine, leading to an increased serum creatinine. This effect is due to inhibition of the organic cation transporter 2.25
Drug interactions with dolutegravir have been investigated. Co-administration with an antacid resulted in a 30% reduction in dolutegravir AUC and 70% reduction in Cmax. There was no significant effect on dolutegravir when combined with tenofovir, with only a slight increase in tenofovir exposure. There is no effect of dolutegravir on lopinavir/ritonavir or darunavir/ritonavir; however ,there was reduction in both AUC (22%) and Cmax (11%) when coadministered with darunavir/ritonavir. Both boosted and unboosted atazanavir resulted in an increase in dolutegravir concentrations, with no effect on atazanavir itself. Etravirine significantly reduced dolutegravir concentrations (AUC 71%, Cmax 52%), but was attenuated with the addition of darunavir/ritonavir.24
Recent phase 3 studies (Table 2) with dolutegravir showed that it was well tolerated and exhibited superiority over efavirenz/emtricitabine/tenofovir26 and noninferiority to raltegravir27 in treatment-naive patients. Dolutegravir was also shown to be well tolerated in treatment experienced patients28 and showed superiority when compared to raltegravir.29
MK-1439
This novel NNRTI was shown to be active given once daily in HIV-positive patients.30 MK-1439 is metabolized by CYP3A4/5 and was shown to neither induce nor inhibit CYP3A metabolism. It has activity against the most prevalent NNRTI-resistant viruses,31 and the elimination half-life of 10 to 16 hours in HIV-positive patients supports once daily dosing.30
Tenofovir alafenamide
Tenofovir alafenamide (TAF) is a novel oral bioavailable prodrug of tenofovir (TDV), which exhibits antiretroviral activity against reverse transcriptase.32 The currently available agent tenofovir disoproxil (TDF, Viread®) is also a prodrug to TDV. Both agents are converted by different pathways to the active parent compound, TDV.33 At much lower doses, TAF has been shown to have superior efficacy compared to TDF 300 mg, with approximately 80% to 97% lower plasma TDV concentrations and higher intracellular concentrations.32 TAF has been studied in combination with other agents (emtricitabine, elvitegravir, and cobicistat) as a single-tablet regimen34 and in combination with emtricitabine, darunavir, and cobicistat.35
HIV PREVENTION AND EARLY DETECTION
Pre-exposure prophylaxis (PrEP)
In July 2012 the FDA approved once daily use of the combination of emtricitabine/tenofovir disoproxil (Truvada®) for PrEP in sexually active adults at high risk for acquiring HIV.36 It is the first drug to be approved for this indication. Patients need to be counseled that taking this medication does not replace safe sexual practices to avoid acquiring HIV.37 A number of studies have been conducted to support the use of tenofovir in PrEP (Table 3). Studies were conducted internationally in both men who have sex with men (MSM) and heterosexual populations. HIV reduction rates with the use of tenofovir or emtricitabine/tenofovir compared to placebo ranged from 44% to 84%.38−40 One study conducted in African women was stopped due to lack of efficacy likely due to low adherence rates.41 Based on these trial results, the Centers for Disease Control and Prevention has published interim guidance for the use of PrEP for MSM and heterosexual populations.42,43
Home HIV testing
Although there are home HIV tests available for the public, OraQuick®, approved in July 2012, is the first over-the-counter test for HIV designed for confidential in-home testing with results within 20 minutes. Through an oral swab of the upper and lower gums of the mouth, the test will detect the presence of HIV-1 and HIV-2 antibodies. Researchers compared the results of the OraQuick® In-Home HIV Test with laboratory tests performed by a trained professional on 4,999 people. The laboratory results showed 96 people tested HIV-positive and 4,903 were HIV negative. In comparison, the test was 99.9% effective in reporting negative results with 1 false positive. Additionally, 91.7% of the HIV-positive participants were correctly identified with this test.44
As a positive test result indicates that the patient may have HIV, a consult and confirmatory test is needed with a healthcare professional. It may take antibodies up to 3 months to develop, so it is recommended that patients with a negative result repeat the test at least 3 months after the last ‘risk’ event.45
CONCLUSION
The many significant advances in the treatment of HIV that have occurred in the past few years have had a major impact on patient survival. Newly developed, highly active antiretroviral therapies and regimens allow for single-tablet, once daily dosing or significantly reduce overall pill burden, which show promise for improving patient adherence to prescribed medication therapy. Strict adherence is vital for suppression of viral loads and to slow progression of disease and development of resistant virus. The availability of at-home testing may lead to an increase in the number of HIV diagnoses among the approximately 1 in 5 patients who are unaware of their infection status. Pre-exposure prophylaxis of uninfected sexual partners of HIV-positive patients has been shown to decrease transmission rates given medication therapy is strictly adhered to. Medications currently in development show promise in giving providers and patients more options to help maintain progression-free survival. It is difficult to predict advances in diagnosis and therapy that may occur over the coming decade-however, if the previous decade provides any insight, we can expect many significant and life-enhancing advances for our patients.
Table 1. Recommendations for initiating antiretroviral therapy in treatment-naive patients
4
Recommendation
Strength*
AI
AII
BIII
AI
AI
AIII
AI
AII
AII
* Footnote: Rating for recommendations
Strength of recommendation
Quality of evidence
Table 2. Phase 3 studies comparing dolutegravir
Study
Dolutegravir
Comparator
Efficacy
Safety
Other
-
Abbreviations: AEs, adverse events; BID, twice daily; BR, background regimen; DTG, dolutegravir; EFV, efavirenz; GI, gastrointestinal; INSTI, integrase strand transfer inhibitor; QD, once daily; RAL, raltegravir.
Table 3. Recent studies utilizing tenofovir or emtricitabine/tenofovir for preexposure prophylaxis
Study
Patient population
Medication
HIV reduction rate compared to placebo
44%
62%
Study stopped due to lack of efficacy
Abbreviations: FTC/TDF, emtricitabine/tenofovir; MSM, men who have sex with men; PrEP, pre-exposure prophylaxis; QD, once daily; TDF, tenofovir.
REFERENCES
1. Kaiser Family Foundation. US Global Health Policy. Fact sheet: the Global HIV /AIDS Epidemic. December 2012. Available at: http://kaiserfamilyfoundation.files.wordpress.com/2013/01/3030-17.pdf Accessed June 15, 2013.
2. Kaiser Family Foundation. Fact sheet: the HIV/AIDS epidemic in the United States. HIV/AIDS Policy. March 2013. Available at: http://www.kff.org/hivaids/upload/3029-14.pdf. Accessed June 15, 2013.
3. U.S. Centers for Disease Control and Prevention. National Center for HIV/AIDS, Viral Hepatitis, STD & TB Prevention. Division of HIV/AIDS Prevention. Trends in annual rates of death due to the 9 leading causes among persons 25−44 years old, United States, 1987-2009. Available at:
http://www.cdc.gov/hiv/pdf/statistics_surveillance_statistics_slides_HIV_mortality.pdf. May 2012. Accessed February 23, 2013.
4. FDA. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1−infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed Dec 9, 2012.
5. Lima VD, Harrigan R, Murray M, et al. Differential impact of adherence on long-term treatment response among naive HIV-infected individuals. AIDS. 2008;22:2371−2380.
6. Kakuda TN. Human immunodeficiency virus infection. In: Talbert RL et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 8th ed. New York: McGraw-Hill; 2011:2169−2190.
7. Viread (tenofovir disoproxil fumarate) [package insert]. Foster City, CA: Gilead Sciences, Inc.; November 2012.
8. Viramune (nevirapine) [package Insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; November 2012.
9. Mills A, Antinori A, Clotet B, et al, on behalf of the ECHO and THRIVE study groups. Neurological and psychiatric tolerability of rilpivirine (TMC278) vs. efavirenz in treatment-naïve, HIV-1−infected patients at 48 weeks. HIV Med. 2013 Jan 9. doi: 10.1111/hiv.12012 [Epub ahead of print].
10. Emmelkamp JM, Rockstroh JK. CCR5 antagonists: comparison of efficacy, side effects, pharmacokinetics and interactions-review of the literature. Eur J Med Res. 2007;12:409−417.
11. Selzentry (maraviroc) [package insert]. Research Triangle Park, NC: ViiV Healthcare; February 2013.
12. Ayoub A, Alston S, Goodrich J, et al. Hepatic safety and tolerability in the maraviroc clinical development program. AIDS. 2010;24:2743−2750.
13. Walmsley S, Campo R, Goodrich J, et al. Low risk of malignancy with maraviroc in treatment-experienced and treatment-naïve patients across the maraviroc clinical development program. From the XVIII International AIDS Conference, July 18-23, 2010; Vienna, Austria. Abstract TUPE0157.
14. Fuzeon (enfuvirtide) [package insert]. South San Francisco, CA: Genentech, Inc. and Trimeris, Inc.; 2012.
15. Lu J, Deeks SG, Hoh R, et al. Rapid emergence of enfuvirtide resistance in HIV-1−infected patients: results of a clonal analysis. J Acquir Immune Defic Syndr. 2006;43:60−64.
16. Isentress (raltegravir) [package insert]. Whitehouse Station, NJ: Merck & Co Inc.; 2012.
17. Lee FJ, Amin J, Bloch M, Pett S, Marriott D, Carr A. Skeletal muscle toxicity associated with raltegravir-based combination antiretroviral therapy in HIV-infected adults. J Acquir Immune Defic Syndr. 2013;62:525−533.
18. Stribild (elvitegravir, cobicistat, emtricitabine, tenofovir disoproxil fumarate) [package insert]. Foster City, CA: Gilead Sciences, Inc.; August 2012.
19. FDA. Antiretroviral drugs used in the treatment of HIV Infection. Available at: http://www.fda.gov/ForConsumers/byAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm118915.htm. Accessed February 10, 2013.
20. Cohen CJ, Molina JM, Cahn P, et al, on behalf of the ECHO and THRIVE Study Groups. Efficacy and safety of rilpivirine (TMC278) versus efavirenz at 48 weeks in treatment-naïve HIV-1−infected patients: pooled results from the phase 3 double-blind randomized ECHO and THRIVE Trials. J Acquir Immune Defic Syndr. 2012;60:33−42.
21. Edurant (rilpivirine) [package insert]. Titusville, NJ: Janssen Therapeutics; December 2012.
22. Complera (emtricitabine/rilpivirine/tenofovir disoproxil fumarate) [package insert]. Foster City, CA: Gilead Sciences, Inc.; January 2013.
23. Rimsky L, Vingerhoets J, Van Eygen V, et al. Genotypic and phenotypic characterization of HIV-1 isolates obtained from patients on rilpivirine therapy experiencing virologic failure in the phase 3 ECHO and THRIVE studies: 48-week analysis. J Acquir Immune Defic Syndr. 2012;59:39−46.
24. Lenz JCC, Rockstroh JK. S/GSK1439572, a new integrase inhibitor for the treatment of HIV: promises and challenges. Expert Opin Investig Drugs. 2011;20:537−548.
25. Koteff J, Borland J, Chen S, et al. A phase 1 study to evaluate the effect of dolutegravir on renal function via measurement of iohexol and para-aminohippurate clearance in health subjects. Br J Clin Pharmacol. 2013;75:990−996.
26. Walmsley S, Antela A, Clumeck N, et al. Dolutegravir (DTG: S/GSK 1349572) + abacavir/lamivudine once daily statistically superior to tenofovir/emtricitabine/efavirenz: 48-week results – SINGLE (ING114467). From the 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), September 9−12, 2012, San Francisco, CA. Abstract H-556b.
27. Raffi F, Rachlis A, Stellbrink HJ, et al, on behalf of the SPRING-2 study group. Once-daily dolutegravir versus raltegravir in antiretroviral-naïve adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381:735−743.
28. Nichols G, Mills A, Grossberg R, et al. Antiviral activity of dolutegravir in subjects with failure on an integrase inhibitor-based regimen: week 24 phase 3 results from VIKING-3. From the 11th International Congress on Drug Therapy in HIV Infection, November 11−15, 2012; Glasgow, United Kingdom. Abstract O232.
29. Pozniak A, Mingrone H, Shuldyakov A, et al. Dolutegravir (DTG) versus raltegravir (RAL) in ART-experienced, integrase-naïve subjects: 24-week interim results from SAILING (ING111762). From the 20th Conference on Retroviruses and Opportunistic Infections, March 3−6, 2013; Atlanta, GA. Abstract 179LB.
30. Anderson M, Gilmartin J, Robberechts M, et al. Safety and antiviral activity of MK-1439, a novel NNRTI, in treatment-naïve HIV+ patients. From the 20th Conference on Retroviruses and Opportunistic Infections, March 3−6, 2013; Atlanta, GA. Abstract 100.
31. Anderson MS, Gilmartin J, Cilissen C, et al. Safety, tolerability, and pharmacokinetics of single and multiple doses of MK-1439, a novel HIV non-nucleoside reverse transcriptase inhibitor in healthy subjects. From the 20th Conference on Retroviruses and Opportunistic Infections, March 3−6, 2013; Atlanta, GA. Abstract J-163.
32. Ruane P, DeJesus E, Berger D, et al. GS-7340 25 mg and 40 mg demonstrate superior efficacy to tenofovir 300 mg in a 10-day monotherapy study of HIV-1+ patients. From the 19th Conference on Retroviruses and Opportunistic Infections, March 5−8, 2012; Seattle, WA. Abstract 103.
33. Birkus G, Bam RA, Frey CR, et al. Intracellular activation pathways for GS-7340 and the effect of antiviral protease inhibitors. From the 19th Conference on Retroviruses and Opportunistic Infections, March 5−8, 2012; Seattle, WA. Poster 577.
34. Zolopa A, Ortiz R, Sax P, et al. Comparative study of tenofovir alafenamide vs. tenofovir disoproxil fumarate, each with elvitegravir, cobicistat, and emtricitabine, for HIV treatment. From the 20th Conference on Retroviruses and Opportunistic Infections, March 3−6, 2013; Atlanta, GA. Paper 99LB.
35. Gilead Sciences. Gilead’s once-daily novel prodrug for the treatment of HIV meets 24-week primary objective in phase 2 study. Press release, Oct. 31, 2012. Available at: http://investors.gilead.com/phoenix.zhtml?c=69964&p=irol-newsArticle&ID=1752101&highlight=. Accessed March 25, 2013.
36. Truvada (emtricitabine/tenofovir disoproxil fumarate) [package insert]. Foster City, CA: Gilead Sciences, Inc.; July 2012.
37. FDA. Truvada approved to reduce the risk of sexually transmitted HIV in people who are not infected with the virus. Nov. 7, 2012. Available at: http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm312264.htm. Accessed March 1, 2013.
38. Grant RM, Lama JR, Anderson P, et al, for the iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587−2599.
39. Baeten JM, Donnell D, Ndase D, et al, for the Partners PrEP Study Team. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399−410.
40. Thigpen MC, Kebaabetswe PM, Paxton LA, et al; TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423−434.
41. Van Damme L, Corneli A, Ahmed K, et al; FEM-PrEP Study Group. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411−422.
42. U.S. Centers for Disease Control and Prevention. Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65−68.
43. U.S. Centers for Disease Control and Prevention. Interim guidance for clinicians considering the use of preexposure prophylaxis for the prevention of HIV infection in heterosexually active adults. MMWR. 2012;61:586−589.
44. OraSure Technologies Inc. OraQuick Educational Guide for Healthcare Professionals. Available at: http://www.oraquick.com/assets/base/oraquickfull/pdf/8453_02_MultiPager_L.pdf Accessed June 15, 2013.
45. OraQuick In Home HIV Test [package insert]. Bethlehem, PA: OraSure Technologies, Inc; June 2012.
FDA Issues Complete Response Letter for Pz-Cel to Treat Epidermolysis Bullosa
April 22nd 2024Prademagene zamikeracel is a cell therapy designed to incorporate the functional collagen-producing COL7A1 gene into a patient’s own skin cells. The FDA is asking for additional information on manufacturing practices.
