- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Dabigatran: A new orally available anticoagulant for prevention of strokes and thrombosis in patients with atrial fibrillation
Dabigatran etexilate (Pradaxa) was approved by FDA for the prevention of stroke and thrombosis in patients with atrial fibrillation.

Key Points
Abstract
Atrial fibrillation (AF) is the most common clinically significant cardiac arrhythmia in the United States and increases the risk of stroke 4.5-fold. Stroke can result in substantial morbidity, mortality, decreased quality of life, and related healthcare costs. Vitamin K antagonists such as warfarin are effective for stroke prevention in AF, but have several drawbacks. There is a need for new anticoagulant agents that are effective, safe, and convenient to use. Dabigatran etexilate (Pradaxa) is an orally administered reversible direct thrombin inhibitor approved by FDA on October 19, 2010, for the prevention of stroke and thrombosis in patients with AF. In clinical studies, it was given orally by fixed once- or twice-daily dosing, does not require dietary adjustments or routine laboratory monitoring, and is not expected to have cytochrome P450-dependent drug interactions, although it does have P-glycoprotein interactions. In the RE-LY trial, in patients with AF and at least 1 other risk factor for stroke (average CHADS2 score 2.1), dabigatran etexilate 150 mg twice daily was superior to warfarin at preventing the combination of stroke and systemic embolic events with a similar rate of major bleeding. (Formulary. 2011;46:44-53.)
Atrial fibrillation (AF) is the most common clinically significant cardiac arrhythmia.1 In 2000, it was estimated that approximately 2.3 million people in the United States suffered from AF, with the risk of AF increasing with age.1 While the prevalence of AF is less than 1% in people younger than 55 years of age, it rises to 9% in individuals over 80 years of age. Moreover, the progressive aging of the population is expected to increase the worldwide burden of AF dramatically over the next 50 years.1 The increased risk of stroke and risk of systemic embolism are major adverse consequences of the arrhythmia.2 Compared with ischemic stroke due to other causes, strokes associated with AF tend to be more severe and are associated with higher mortality, greater disability, and higher healthcare costs.3,4
Dabigatran etexilate (Pradaxa), manufactured by Boehringer Ingelheim, is an orally available reversible direct thrombin inhibitor (DTI) that was recently approved by FDA to reduce the risk of stroke and systemic embolism in patients with AF.10 It is a promising new oral anticoagulant that may become an attractive alternative to warfarin and may have a significant impact on the current approach to managing stroke prevention in AF.
CHEMISTRY AND PHARMACOLOGY
Dabigatran etexilate is a prodrug of dabigatran, a potent, nonpeptide, small molecule that specifically and reversibly binds to the active site of thrombin and inhibits both free and clot-bound thrombin.11 The structural design is based on the peptide-like thrombin inhibitor N-2-naphthyl-sulfonyl-glycyl-d-para-amidino-phenyl-alanyl-piperidine (NAPAP).12 After oral administration, dabigatran etexilate is rapidly hydrolyzed by nonspecific esterases in plasma and the liver to the active form, dabigatran.10,14 The conversion of dabigatran etexilate to dabigatran is not dependent on the CYP450 enzyme system, and, therefore, dabigatran etexilate has a low potential for interacting with drugs metabolized via CYP450 pathways. Dabigatran etexilate has shown moderate affinity to efflux transporter P-glycoprotein (P-gp), which could lead to increased bioavailability upon co-administration of P-gp inhibitors or reduced bioavailability upon intake of P-gp inducers.13 The pharmacodynamic characteristics and low propensity for food and drug interactions of dabigatran result in it being an oral anticoagulant with fewer management challenges.15,16
PHARMACOKINETICS
Dabigatran etexilate is rapidly absorbed and completely converted to its active form, dabigatran.17 In healthy subjects, peak plasma concentration (Cmax) of dabigatran is achieved within 2 hours after oral dosing.14 It has a half-life of 12 to 17 hours, which permits once-daily or twice-daily oral administration, and has a mean absolute bioavailability of approximately 6.5%, which is not clinically significantly influenced by co-administration with food.14,15,18 Food prolongs the time to peak plasma concentration (Tmax) by approximately 2 hours with no clinically meaningful influence on area under the curve (AUC), total exposure, and Cmax.16 Co-administration with proton pump inhibitors reduces overall drug exposure by 20% to 25%, which is considered to be clinically insignificant and therefore does not necessitate dose adjustment.16 Steady-state concentrations for dabigatran are achieved approximately 3 days after multiple dose administration.16
Dabigatran exhibits plasma protein binding of about 35%, and about 80% of the given dose is excreted by the kidneys.15,16 Exposure to dabigatran has been shown to increase with renal impairment and to correlate with severity of renal dysfunction.19 Dabigatran exposure is 20% to 30% higher in elderly females than in elderly males, possibly due to gender-dependent differences in creatinine clearance, but does not cause any substantial clinical difference.18 Dabigatran pharmacokinetics are not substantially affected by moderate hepatic impairment.20
CLINICAL TRIALS
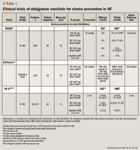
Results of the PETRO trial led to the PETRO-Extension (PETRO-Ex) trial, which was an extended evaluation of patients recruited in the PETRO study. From the PETRO study, 361 patients were rolled over to the PETRO-Ex study, which assessed only the patients who were treated with dabigatran etexilate; the warfarin arm was not continued.22 All patients were initially maintained on the same dabigatran etexilate doses as in PETRO, except for patients treated with the 50-mg twice-daily dose, who were switched to 150 mg once daily.22 Thromboembolic event rates were lowest in the dabigatran etexilate 150-mg and 300-mg twice-daily groups. Major bleeding was most frequent in the 300-mg twice-daily group. Therefore, the 150-mg twice-daily dosage was chosen for further investigation as part of a larger phase 3 trial for stroke prevention in AF, the Randomized Evaluation of Long-term anticoagulant therapY (RE-LY) study.22
The phase 3, prospective, randomized, open-label, blinded end point evaluation (PROBE-design) RE-LY study compared dabigatran etexilate 110 mg and 150 mg twice daily (dosed in a blinded fashion) with dose-adjusted warfarin (dosed in an unblinded fashion with a target INR value of 2.0-3.0).23,24 This was a multicenter trial that enrolled 18,113 patients with AF who were at risk for stroke (mean age, 71 years; 63.6% of whom were men). The risk of stroke in these patients was calculated using the CHADS2 scoring system, in which congestive heart failure, hypertension, age of 75 years or older, and diabetes mellitus are each assigned one point, and previous stroke or transient ischemic attack is assigned 2 points. The mean CHADS2 score (summing all the points) was 2.1.23 About half the patients in RE-LY had received long-term therapy with VKAs before enrollment, a stratum that was prespecified by the protocol.23 The primary outcome of RE-LY was time to first systemic embolism or stroke (including hemorrhagic stroke).23,24
The rates of primary outcome were 1.7% per year in the warfarin group, as compared with 1.5% per year in the group that received dabigatran etexilate 110 mg twice daily (RR with dabigatran etexilate, 0.90; 95% CI, 0.74–1.10; P<.001 for noninferiority) and 1.1% per year in the group that received 150 mg twice-daily dabigatran etexilate (RR, 0.65; 95% CI, 0.52–0.81; P<.001 for superiority).25 The rate of major bleeding was 3.36% per year in the warfarin group, as compared with 2.71% per year in the group receiving 110 mg twice-daily dabigatran etexilate (P=.003) and 3.11% per year in the group receiving 150 mg twice-daily dabigatran etexilate (P=0.31). Interestingly, the increased antithrombotic efficacy of dabigatran did not correlate with increased risk of hemorrhagic stroke or intracranial hemorrhage: rate of hemorrhagic stroke was 0.12% and 0.10% per year in the dabigatran 110-mg and 150-mg twice-daily groups, respectively, compared with 0.38% in the warfarin group (P<.001 for both comparisons). Likewise, rates of intracranial bleeding were higher with warfarin treatment (0.74%) compared with the 110-mg (0.23%) and the 150-mg (0.30%) doses of dabigatran etexilate (P<.001 for both comparisons with warfarin; P=.28 between dabigatran etexilate doses). The mortality rate was 4.13% per year in the warfarin group, as compared with 3.75% per year with dabigatran etexilate 110 mg twice daily (P=.13) and 3.64% per year with dabigatran etexilate 150 mg twice daily (P=.051).23 These results show that in comparison to warfarin, dabigatran etexilate confers a similar or lower risk of stroke and a lower or similar rate of major hemorrhage in patients with AF.
ADVERSE EVENTS
Safety data for dabigatran etexilate (110 mg or 150 mg twice daily) in patients with AF are based on the 18,113-patient RE-LY trial.23 The only adverse event that was significantly more common with dabigatran etexilate than with warfarin was dyspepsia. It occurred in 348 patients out of 6,022 (5.8%) in the warfarin group, 707 patients out of 6,015 (11.8%) in the dabigatran etexilate 110-mg twice daily group, and 688 patients out of 6,076 (11.3%) in the dabigatran etexilate 150-mg twice-daily group (P<.001 for both comparisons). Elevations in the serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level of more than 3 times the upper limit of normal (3x ULN) did not occur more frequently with dabigatran etexilate at either dose than with warfarin.23
DRUG INTERACTIONS
In vitro studies suggest no interaction with CYP isoenzymes, and this is supported by the findings from in vivo studies in healthy volunteers given dabigatran etexilate and inhibitors of CYP2C9 and CYP3A4.14,26,27 Dabigatran etexilate has a moderate affinity to P-gp. When a P-gp inducer, rifampin 600 mg once daily for 7 days was followed by a single dose of dabigatran, it decreased its AUC and Cmax by 66% and 67%, respectively. After cessation of rifampin on Day 7, dabigatran exposure was close to normal.10 No interaction is observed when dabigatran etexilate is co-administered with digoxin, a substrate of P-gp; however, co-administration with amiodarone, a P-gp inhibitor, increased dabigatran bioavailability by approximately 50% to 60% in a phase 1 interaction study.13,28 Dabigatran etexilate has not been evaluated with propafenone at this point, nor has it been evaluated with the P-gp inducer St John's wort, which has also been shown to reduce serum digoxin concentrations.29 In clinical studies exploring CYP3A4, CYP2C9, P-gp and other pathways, dabigatran did not meaningfully alter the pharmacokinetics of amiodarone, atorvastatin, clarithromycin, diclofenac, clopidogrel, digoxin, pantoprazole, or ranitidine.10
DOSING AND ADMINISTRATION
For patients with creatinine clearance (CrCl) >30 mL/min, the recommended dose of dabigatran etexilate is 150 mg taken orally, twice daily, with or without food. For patients with CrCl 15 to 30 mL/min, the recommended dose is 75 mg twice daily. Dosing recommendations for patients with a CrCL <15 mL/min or those on dialysis cannot be provided.
The package insert for Pradaxa states that patients should swallow the capsules whole. Breaking, chewing, or emptying the contents of the capsule can result in increased exposure. If a dose of dabigatran etexilate is not taken at the scheduled time, the dose should be taken as soon as possible on the same day; the missed dose should be skipped if it cannot be taken at least 6 hours before the next scheduled dose. The dose should not be doubled to make up for a missed dose.10
When converting patients from warfarin therapy to dabigatran, discontinue warfarin and start dabigatran when the international normalized ratio (INR) is below 2.0. When converting from dabigatran to warfarin, adjust the starting time of warfarin based on creatinine clearance. For CrCl >50 mL/min, start warfarin 3 days before discontinuing dabigatran; for CrCl 31 to 50 mL/min, start warfarin 2 days before discontinuing dabigatran; for CrCl 15 to 30 mL/min, start warfarin 1 day before discontinuing dabigatran; for CrCl <15 mL/min, no recommendations are made. Because dabigatran can contribute to an elevated INR, the INR will better reflect warfarin's effect after dabigatran has been stopped for at least 2 days.10
For patients currently receiving a parenteral anticoagulant, start dabigatran 0 to 2 hours before the time that the next dose of the parenteral drug was to have been administered or at the time of discontinuation of a continuously administered parenteral drug (eg, intravenous unfractionated heparin). For patients currently taking dabigatran, wait 12 hours (CrCl ≥30 mL/min) or 24 hours (CrCl <30 mL/min) after the last dose of dabigatran etexilate before initiating treatment with a parenteral anticoagulant.10
If possible, discontinue dabigatran 1 to 2 days (CrCl ≥50 mL/min) or 3 to 5 days (CrCl <50 mL/min) before invasive or surgical procedures because of the increased risk of bleeding. Consider longer times for patients undergoing major surgery, spinal puncture, or placement of a spinal or epidural catheter or port in whom complete hemostasis may be required. If surgery cannot be delayed, there is an increased risk of bleeding. Bleeding risk can be assessed by the ecarin clotting time (ECT). This test is a better marker of the anticoagulant activity of dabigatran than activated partial thromboplastin time (aPTT), prothrombin time (PT)/INR, or thrombin time (TT). If ECT is not available, the aPTT test provides an approximation of anticoagulant activity.10
FORMULARY CONSIDERATIONS
Dabigatran etexilate, a potent orally administered DTI, is a promising alternative to warfarin. It has been given in clinical trials at a fixed twice-daily dose for stroke prevention in AF. It does not require routine coagulation monitoring or dietary adjustments (eg, avoidance of green leafy vitamin K–containing vegetables) and it does not have CYP450 drug interactions. It is possible that these factors may improve quality of life for patients requiring anticoagulation and reduce clinician and caregiver burden but conclusive studies to evaluate this have not been conducted.
In the RE-LY trial, in patients with AF and an average CHADS2 score of 2.1, dabigatran etexilate 150-mg twice daily was superior to warfarin at preventing stroke with a comparable risk of major bleeding.22 In patients receiving 110-mg twice-daily dose of dabigatran etexilate, the rate of stroke or systemic embolism was similar to that with warfarin, with a lower rate of major bleeding events.22 This smaller dose is not recommended in the package insert and the 110-mg tablet size is not available.
Dabigatran etexilate capsules should not be crushed precluding administration to those with the inability to swallow. Dabigatran etexilate is a substrate for the P-gp enzyme system and interacted with both a P-gp inducer (rifampin) and P-gp inhibitor (amiodarone).10,30 Given the 50% to 67% alteration in dabigatran blood concentrations with P-gp inducers and inhibitors and the inability to crush or extemporaneously compound the medication, avoiding concurrent dabigatran etexilate therapy with rifampin and amiodarone seems prudent.10 Future research to identify the extent of interaction with further P-gp inhibitors or inducers is critically needed, but in the interim it seems prudent to avoid use with other P-gp inducers and inhibitors. This can be problematic since verapamil, propafenone, and dronedarone are also P-gp inhibitors that are commonly used in patients with AF.
Dabigatran etexilate should not be used in patients with severe renal dysfunction (CrCl <15 mL/min) or those on dialysis. For patients with CrCl 15 to 30 mL/min, the recommended dose is 75 mg twice daily.10 As such, more diligent assessment of renal function in those with renal insufficiency or dysfunction or those at high risk of developing such a disorder (such as patients receiving IV contrast or those with nephropathy) seems prudent. In patients with moderate hepatic impairment (Child-Pugh classification B), the pharmacokinetic/pharmacodynamic or safety profile of dabigatran etexilate is not clinically affected but caution is needed in severe hepatic dysfunction.19
Vitamin K, though nonspecific in its action, can reverse the pharmacologic effect of warfarin over time. However, no specific antidote has been confirmed for use with dabigatran etexilate.31 The need for rapid reversal of the effects of dabigatran could occur with severe bleeding, overdose, and emergency surgery. Because the anticoagulant effect of dabigatran etexilate is less influenced by exogenous factors and it has a shorter duration of effect than warfarin, there is likely a reduced need for reversal agents under normal circumstances, such as elective surgery in patients with normal renal function.15,16,20
According to an article in Cardiobrief, dabigatran etexilate will cost about $237 a month at the drugstore counter.32 A representative from Boehringer Ingelheim, the manufacturer of dabigatran etexilate, said that the wholesale acquisition cost of the drug will be $6.75 per day, which includes both the 150-mg and the 75-mg capsules.32 Thus, the cost of dabigatran etexilate is higher than warfarin but may require fewer laboratory monitoring–related costs. It may also reduce costs due to prevention of stroke and bleeding, which would be disease specific; however, there are currently no published pharmacoeconomic analyses. In essence, it seems like the costs of managing anticoagulation will be shifted from office care when warfarin is used to prescription drug cost when dabigatran is used.
In conclusion, dabigatran etexilate is a promising new oral anticoagulant that offers a viable therapeutic alternative to warfarin. However, there are some patients for which dabigatran etexilate therapy should not be used and some cautions to consider.
Dr Talati is senior scientist, Hartford Hospital, Hartford, Conn., and University of Connecticut, Storrs. Dr White is professor of pharmacy, University of Connecticut, Storrs, and director, UCONN/Hartford Hospital, Evidence-based Practice Center, Hartford, Conn.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, medical editor, Department of Pharmacy Services, University of Connecticut/Hartford Hospital, Evidence-based Practice Center, Hartford, Conn., and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn; and by Craig I. Coleman, PharmD, associate professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
EDITORS' NOTE: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
REFERENCES
1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375.
2. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154(13):1449–1457.
3. Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27(10):1760–1764.
4. Wolf PA, Mitchell JB, Baker CS, Kannel WB, D'Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med. 1998;158(3):229–234.
5. Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):454S–545S.
6. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867.
7. Cios DA, Baker WL, Sander SD, Phung OJ, Coleman CI. Evaluating the impact of study-level factors on warfarin control in US-based primary studies: a meta-analysis. Am J Health-Syst Pharm. 2009;66(10):916–925.
8. Nichol MB, Knight TK, Dow T, et al. Quality of anticoagulation monitoring in nonvalvular atrial fibrillation patients: comparison of anticoagulation clinic versus usual care. Ann Pharmacother. 2008;42(1):62–70.
9. Connolly SJ, Pogue J, Eikelboom J, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118(20):2029–2037.
10. Pradaxa (dabigatran etexilate). Summary of product characteristics. Available at: http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed December 7, 2010.
11. Baetz BE, Spinler SA. Dabigatran etexilate: an oral direct thrombin inhibitor for prophylaxis and treatment of thromboembolic diseases. Pharmacotherapy. 2008;28(11):1354–1373.
12. Hauel NH, Nar H, Priepke H, Ries U, Stassen JM, Wienen W. Structure-based design of novel potent nonpeptide thrombin inhibitors. J Med Chem. 2002;45(9):1757–1766.
13. Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36(2):386–399.
14. Ufer M. Comparative efficacy and safety of the novel oral anticoagulants dabigatran, rivaroxaban and apixaban in preclinical and clinical development. Thromb Haemost. 2010;103(3):572–585.
15. Stangier J, Rathgen K, Stähle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64(3):292–303.
16. Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008;47(5):285–295.
17. Stangier J, Eriksson BI, Dahl OE, et al. Pharmacokinetic profile of the oral direct thrombin inhibitor dabigatran etexilate in healthy volunteers and patients undergoing total hip replacement. J Clin Pharmacol. 2005;45(5):555–563.
18. Stangier J, Stähle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet. 2008;47(1):47–59.
19. Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49(4):259–268
20. Stangier J, Stähle H, Rathgen K, Roth W, Shakeri-Nejad K. Pharmacokinetics and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor, are not affected by moderate hepatic impairment. J Clin Pharmacol. 2008;48(12):1411–1419.
21. Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol. 2007;100(9):1419–1426.
22. Nagarakanti R, Ezekowitz MD, Parcham-Azad K, et al. Abstract 4629: Long-term open label extension of the prevention of embolic and thrombotic events on dabigatran in atrial fibrillation (PETRO-Ex study). Circulation. 2008;118:S_922.
23. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151.
24. Ezekowitz MD, Connolly S, Parekh A, et al. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157(5):805–810, e2.
25. FDA. Clinical review for NDA 022-512 Pradaxa (Dabigatran). Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM226011.pdf. Accessed December 8, 2010
26. Stangier J, Rathgen K, Stahle H, Reseski K, Kornicke T, Roth W. Coadministration of dabigatran etexilate and atorvastatin: assessment of potential impact on pharmacokinetics and pharmacodynamics. Am J Cardiovasc Drugs. 2009;9(1):59–68.
27. Stangier J, Stähle H, Rathgen K, Reseski K, Kornicke T. Coadministration of the oral direct thrombin inhibitor dabigatran etexilate and diclofenac has little impact on the pharmacokinetics of either drug. J Thromb Haemost. 2007;5(suppl 2):abstract P-T-677.
28. Stangier J, Stähle H, Rathgen K, Reseski K, Kornicke T. No interaction of the oral direct thrombin inhibitor dabigatran etexilate and digoxin. J Thromb Haemost. 2007;5(suppl 2):abstract P-W.672.
29. Gurley BJ, Swain A, Williams DK, Barone G, Battu SK. Gauging the clinical significance of P-glycoprotein-mediated herb-drug interactions: comparative effects of St. John's wort, Echinacea, clarithromycin, and rifampin on digoxin pharmacokinetics. Mol Nutr Food Res. 2008;52(7):772–779.
30. Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost. 2009;(suppl 1):9s–16s.
31. Lubetsky A, Yonath H, Olchovsky D, Loebstein R, Halkin H, Ezra D. Comparison of oral vs intravenous phytonadione (vitamin K1) in patients with excessive anticoagulation: a prospective randomized controlled study. Arch Intern Med. 2003;163(20):2469–2473.
32. Cardiobrief. Pradaxa (Dabigatran) pricing start to emerge. Available at: http://cardiobrief.org/2010/10/26/pradaxa-dabigatran-pricing-starts-to-emerge/. Accessed December 10, 2010.
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
