- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Dapoxetine: A novel, fast-acting serotonin reuptake inhibitor
Dapoxetine (Alza/Ortho-McNeil) is a novel oral medication undergoing FDA review for premature ejaculation, one of the most common disorders of sexual dysfunction in men. Dapoxetine is a fast-acting inhibitor of the serotonin reuptake transporter. It has a short half-life and is structurally related to the antidepressant fluoxetine, allowing for on-demand dosing.
Abstract Dapoxetine (Alza/Ortho-McNeil) is a novel oral medication undergoing FDA review for premature ejaculation, one of the most common disorders of sexual dysfunction in men. Dapoxetine is a fast-acting inhibitor of the serotonin reuptake transporter. It has a short half-life and is structurally related to the antidepressant fluoxetine, allowing for on-demand dosing. Recent phase 3 clinical trials in patients with premature ejaculation have shown dapoxetine to be effective in improving the time to ejaculation without any major adverse events, except nausea. If approved, dapoxetine would be the first drug specifically indicated for premature ejaculation, and it may offer patients a safer and more tolerable alternative compared to traditional selective serotonin reuptake inhibitors (SSRIs), which are currently used off-label to treat this condition. (Formulary. 2005;40:227–230.)
Sexual dysfunction is characterized by a disturbance in the processes that characterize the sexual response cycle, which can be broken down into 4 phases: disturbances in desire, excitement, orgasm, and resolution.1 Premature ejaculation (PE) is a problem associated with the orgasm phase of the sexual cycle. PE is the most common form of sexual dysfunction in men, affecting up to 21% of men aged 18 to 59 years in the United States.2 According to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), PE is defined as persistent or recurrent ejaculation with minimal sexual stimulation before, on, or shortly after penetration and before the person wishes it. The diagnostic criteria also include the emotional and interpersonal impact of PE.1

CHEMISTRY AND PHARMACOLOGY Dapoxetine ((+)-(S)-N,N-dimethyl-(α)-[2(1-naphthalenyloxy)ethyl]-benzenemethanamine hydrochloride) is a selective serotonin (5-HT) reuptake inhibitor, similar in structure to fluoxetine.5 The SSRIs and dapoxetine appear to be effective in premature ejaculation due to the critical role of serotonin in the pathophysiology of the disease.

The pathophysiology associated with PE includes a reduction in the serotonergic neurotransmission, 5-HT2c receptor hyposensitivity, and/or 5-HT1a receptor hypersensitivity.5 Studies in laboratory animals have shown that the administration of SSRIs actively blocks presynaptic membranes of 5-HT transporters, resulting in higher serotonin levels in the synaptic cleft. The serotonin then binds to 5-HT2c and 5-HT1a receptors to delay ejaculation. Dapoxetine exhibits its efficacy by primarily inhibiting the reuptake of the serotonin transporter. It was also shown to bind and inhibit the reuptake transporters of dopamine and norepinephrine.7

Dapoxetine undergoes hepatic metabolism to 2 metabolites, desmethyldapoxetine and didesmethyldapoxetine, both of which have much lower plasma concentrations compared with dapoxetine. Information on specific isoenzymes involved in metabolism and volume of distribution and details on excretion had not been published at press time.8
CLINICAL TRIALS The clinical data available on dapoxetine are limited to one phase 2 and two phase 3 trials, all published in abstract form.
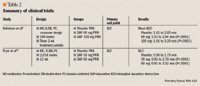
Hellstrom et al9 randomized 166 adult heterosexual men in a multicenter, placebo-controlled, double-blind, 3-period, crossover phase 2 study to either dapoxetine 60 mg, dapoxetine 100 mg, or placebo. All patients underwent a 2-week screening period followed by three 2-week treatment phases, each separated by a 72-hour washout period. Each drug was to be taken 1 to 2 hours prior to anticipated sexual activity only. Inclusion criteria included men aged 18 to 65 years in a monogamous relationship for at least 6 months and a baseline intravaginal latency time (IELT) of <2 minutes (mean baseline IELT=1.01 minutes). The primary end point was IELT measured by a stopwatch held by the partner.
Nausea was the most commonly reported adverse event, occurring with greater frequency in the dapoxetine 100-mg group (16.1%) compared with the 60-mg group (5.6%) and placebo group (0.7%). It is also important to mention that 9 out of 10 patients who discontinued the study due to adverse events were receiving dapoxetine 100 mg. On the basis of these results, the 60-mg dose was selected to be further evaluated in the phase 3 trials.
Pryor et al10 conducted 2 randomized, double-blind, placebo-controlled, multicenter studies of identical design (reported in 1 abstract) in 2,614 men diagnosed with premature ejaculation according to DSM-IV criteria, one evaluating the 30-mg dose of dapoxetine and the other evaluating the 60-mg dose.Subjects participated in a 2-week baseline period followed by a 12-week treatment period and were randomized to either 30 mg of dapoxetine, 60 mg of dapoxetine, or placebo. Each drug was taken 1 to 3 hours prior to anticipated sexual activity. No other inclusion criteria were specified, except that subjects had a mean intravaginal ejaculatory latency time of 2 minutes. The primary end point was IELT measured with a stopwatch by a partner. Secondary end points included patient perception of Control over Ejaculation and patient perception of Satisfaction with Sexual Intercourse on a 5-point scale (0=very poor to 4=very good). Secondary end points were assessed at baseline, 4, 8, and 12 weeks.
In the studies, both doses of dapoxetine demonstrated significant improvement compared with placebo for the IELT (P<.0001) (Table 2). In comparison, the placebo group went from a baseline of 0.90 to 1.75 minutes. Both the dapoxetine 30-mg dose (P=.0006) and 60-mg dose (P<.001) were more effective than placebo in increasing IELT on the first dose according to the study investigators. Both doses of dapoxetine also demonstrated improvement compared with placebo for the secondary end points. Changes from baseline in the percentage of patients giving ratings of fair, good, and very good for Control over Ejaculation were 2.5% to 51.8% for the 30-mg dapoxetine group, 3.3% to 58.4% for the 60-mg dapoxetine group, and 3.5% to 26.4% for the placebo group. Similar results were seen with Satisfaction of Sexual Intercourse with changes from baseline of 52.4% to 70.9% with 30 mg, 56.7% to 79.2% with 60 mg, and 51.8% to 55.2% with placebo.
Nausea and headache were the most common adverse events reported with both doses of dapoxetine. Nausea was reported by 20% of patients in the 60-mg group and 8.7% of patients in the 30-mg group. Headache was also common in both groups with 5.9% of patients reporting this adverse event in the 30-mg group and 6.8% reporting headache in the 60-mg group. Diarrhea (6.8%) and dizziness (6.2%) were also more common in the 60-mg group compared with both the 30-mg and placebo groups. Discontinuation rates due to adverse events were 0.9% for placebo-treated patients, 4% for those receiving 30 mg of dapoxetine, and 10% for those receiving 60 mg of dapoxetine.
Currently there are no clinical trials or abstracts published comparing dapoxetine with current therapies utilized for premature ejaculation such as the SSRIs, clomipramine, and topical anesthetics.
ADVERSE EVENTS According to a dose-finding analysis, the most common adverse events associated with dapoxetine were nausea, diarrhea, dizziness, and headache, and all 3 were reported to be dose-related.11 The most common adverse events seen in the large phase 3 trial with dapoxetine were nausea and headache. Nausea was fairly significant, with an incidence of 20% reported in patients taking the 60-mg dose of dapoxetine. Overall, 10% of patients discontinued dapoxetine in this group due to adverse events. Dizziness (6.2%) and diarrhea (6.8%) were also fairly common in patients taking 60 mg of dapoxetine. There are currently no reports of any cardiovascular, hepatic, or hematological adverse events associated with dapoxetine.

Due to the lack of current information on renal elimination and specific metabolism pathways, it is too early to speculate on other potential drug interactions.
DOSING AND ADMINISTRATION Based on the recent large phase 3 clinical trial evaluating both efficacy and tolerability, dapoxetine will most likely be marketed in dosages of both 30 and 60 mg. Patients would likely be instructed to take either dosage on an as-needed basis 1 to 3 hours before sexual intercourse. There is no current information on dosage adjustments in patients with either hepatic or renal dysfunction.
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, director of Drug Information Services at Hartford Hospital in Hartford, Conn, and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn; and by Craig I. Coleman, PharmD, assistant professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
EDITORS' NOTE: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
Dr Feret is a clinical assistant professor at the University of Rhode Island College of Pharmacy, Kingston, RI. He can be reached at bferet@uri.edu
REFERENCES 1. Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision). Washington, DC: American Psychiatric Association; 2000.
2. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537–544.
3. Montague DK, Jarow J, Broderick GA, et al, and the AUA Erectile Dysfunction Guideline Update Panel. AUA Guideline on the Pharmacologic Management of Premature Ejaculation. J Urol. 2004;172:290–294.
4. Johnson & Johnson Press Releases & Statements. New Drug Application Submitted for Dapoxetine for Premature Ejaculation. Available at: http:// http://www.jnj.com/news/jnj_news/20041228_115004.htm. Accessed June 20, 2005.
5. Sorbera LA, Castaner J, Castaner RM. Dapoxetine hydrochloride. Drugs Future. 2004;29: 1201–1205.
6. McMahon C. Pharmacological Treatment of Ejaculatory Disorders. J Sex Med. 2004;1(Suppl 1):19–21.
7. Gengo PJ, Giuliano F, McKenna K, et al. Monoaminergic transport binding and inhibition profile of dapoxetine, a medication for the treatment of premature ejaculation [abstract]. Presented at: the 100th American Urological Association Annual Meeting; May 21–26, 2005; San Antonio, Texas. Abstract 878.
8. Dresser M, Lindert K, Lin D, et al. Pharmacokinetics of single and multiple escalating doses of dapoxetine in healthy volunteers [abstract]. Clin Pharmacol Ther. 2004;75(2):113. Abstract P1.
9. Hellstrom W, Gittelman M, Althof S, et al. Dapoxetine HCl for the treatment of premature ejaculation: a phase II, randomized, double-blind, placebo-controlled study [abstract]. J Sex Med. 2004;Suppl 1:59. Abstract 097.
10. Pryor JL, Althof SE, Steidle C, et al. Efficacy and tolerability of dapoxetine in the treatment of premature ejaculation [abstract]. Presented at: the 100th American Urological Association Annual Meeting; May 21–26, 2005; San Antonio, Texas. Abstract 740.
11. Hellstrom W, Althof S, Gittelman M, et al. Dapoxetine for the treatment of men with premature ejaculation (PE):dose-finding analysis [abstract]. Presented at: the 100th American Urological Association Annual Meeting; May 21–26, 2005; San Antonio, Texas. Abstract 877.
12. Dresser M, Jazrawi RP, Desai D, Modi NB. Dapoxetine for the treatment of premature ejaculation: Lack of interaction with PDE5 inhibitors [abstract]. Presented at: the 100th American Urological Association Annual Meeting; May 21–26, 2005; San Antonio, Texas. Abstract 739.
13. Modi NB, Dresser M, Desai D, Jazrawi RP. Dapoxetine for the treatment of premature ejaculation: lack of interaction with ethanol [abstract]. Presented at: the 100th American Urological Association Annual Meeting; May 21–26, 2005; San Antonio, Texas. Abstract 879.
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
