- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Digestive Disease Week 2006: Pharmacologic studies review findings on ACE inhibitors, treatment of Crohn's disease
Focused on a diverse number of areas in the study of gastrointestinal health, Digestive Disease Week (DDW) 2006 in Los Angeles, Calif, yielded important information about the pharmacologic treatment of certain GI cancers and Crohn's disease.
Focused on a diverse number of areas in the study of gastrointestinal health, Digestive Disease Week (DDW) 2006 in Los Angeles, Calif, yielded important information about the pharmacologic treatment of certain GI cancers and Crohn's disease.
Retrospective data suggest ACE inhibitors reduce risk of certain GI cancers
The potential benefit of ACE inhibitors in cancer prevention may be a result of these agents' ability to suppress tumor angiogenesis by blocking vascular endothelial growth factor, Dr Khurana said. This action of ACE inhibitors has been observed in animal and experimental models of cancer.
Dr Khurana's findings were based on data from the Veterans Integrated Service Network database, a registry established in 1996 that contains data on every veteran that received care from the South Central Veterans Administration (VA) Health Care Network from October 1998 to June 2004. Using this database, he assessed the risks of colorectal, esophageal, and pancreatic cancer among veterans who took ACE inhibitors and compared their risk to the risk of veterans who had not been taking ACE inhibitors.
The study included 483,733 patients, 38.4% of whom were considered users of ACE inhibitors for the purpose of this analysis. Patients were placed in the ACE inhibitor group if they were using ACE inhibitors prior to the diagnosis of their cancers.
Among the overall group, 6,697 had colorectal cancer, 659 had esophageal cancer, and 475 had pancreatic cancer.
After adjusting for numerous variables, including reflux disease, alcohol consumption, body mass index, age, smoking, use of nonsteroidal anti-inflammatory drugs (NSAIDs), and diabetes, the use of ACE inhibitors was associated with a 53% reduction in the risk of colorectal cancer (P<.0001), a 52% reduction in the risk of pancreatic cancer (P<.01), and a 46% reduction in the risk of esophageal cancer (P<.01).
"All of the findings were independent of statin use," said Dr Khurana, from the Overton Brooks VA Medical Center in Shreveport, La. Statins also have been associated with reductions in certain cancers, although studies of short duration often fail to find a significant association between statin use and cancer risk reduction, he said.
The results were not stratified by type, dosage, or duration of ACE inhibitor, he said.
A nested case-control study, presented at DDW by George Makar, MD, found a similar effect of ACE inhibitors on colorectal cancer risk. In Dr Makar's study, a database of 339,525 patients with hypertension was used to compare 1,988 cases with colorectal cancer and 18,131 matched controls.
According to Dr Makar, division of gastroenterology at the University of Pennsylvania in Philadelphia, Pa, laboratory data suggest that excess angiotensin II may promote the growth of colorectal cancer by inducing neovascularization and expression of insulin-like growth factor-1 (IGF-1) receptors. ACE inhibitors may have a chemoprotective effect by acting to interrupt the IGF-1 pathway, he said.
Patients with <1 year of ACE inhibitor use prior to a diagnosis of colorectal cancer were excluded from the analysis. The duration of follow-up was 4.5 years.
ACE inhibitor use >3 years was associated with an odds ratio of colorectal cancer of 0.79 (95% CI, 0.64–0.96) compared with no exposure, corresponding to a relative risk reduction of 21%. A significant dose-duration effect was evident: Those who used ACE inhibitors for ≥5 years experienced a 45% reduction in colorectal cancer risk.
"The association was stronger among diabetics, who have a greater baseline risk of colorectal cancer," said Dr Makar.
In patients with diabetes, ≥3 years of ACE inhibitor use was associated with a 73% reduction in the risk of colorectal cancer.
Anti-TNF agents maintain prominent roles in treatment of Crohn's disease
The introduction of anti-tumor necrosis factor (TNF) antibodies has impacted the treatment of inflammatory bowel disease over the past decade, especially that of patients with refractory and/or fistulizing disease. Ongoing advances in the pharmacologic development of these agents will ensure that anti-TNF therapy retains a featured role in the treatment of patients with Crohn's disease, said presenters at DDW 2006.
Anti-TNF therapies modulate the inflammation of Crohn's disease, have a rapid onset of action, have prolonged benefits, and are generally well tolerated. Potential drawbacks of these agents include infusion site reactions, a risk of infection, immunogenicity resulting in subsequent loss of efficacy, and the rare development of lymphomas.
Currently, infliximab is the only anti-TNF agent approved for the treatment of Crohn's disease. With more anti-TNF agents in the pipeline, the eventual choice of agent may come down to safety, cost, and convenience.
A new anti-TNF therapy in phase 3 clinical study is certolizumab pergol, a humanized monoclonal antibody that has a polyethylene glycol molecule added to delay clearance from the body, allowing for an extended interval between doses. The agent has demonstrated success in achieving remission and maintaining clinical response in patients with moderate-to-severe Crohn's disease with once-monthly subcutaneous injection.
Adalimumab, a recombinant human IgG1 antibody already approved for the treatment of rheumatoid arthritis, also has shown efficacy in phase 3 trials of Crohn's disease.
In results from the CHARM (Crohn's trial of the fully Human antibody Adalimumab for Remission Maintenance) study presented at DDW, adalimumab maintained remission out to 56 weeks in patients with moderate-to-severely active Crohn's disease who already had responded to induction therapy with adalimumab.
Seven hundred seventy-eight patients who had been on open-label induction therapy with adalimumab for 4 weeks were randomized to placebo or adalimumab weekly or every other week. At 4 weeks after randomization, 499 achieved a clinical response, defined as a reduction of 70 or more points on the Crohn's Disease Activity Index (CDAI).
These 499 patients became the group for primary efficacy analysis. Among them, 47% of patients receiving weekly adalimumab achieved remission at 26 weeks, defined as achieving a CDAI score of ≤150, compared with 17% of placebo recipients (P<.001). Remission at 52 weeks was achieved by 41% of the patients in the weekly adalimumab group and 12% of the patients in the placebo group (P<.001).
"We believe that adalimumab represents a new maintenance therapy for Crohn's disease," said the study's lead investigator, Jean-Frederic Colombel, MD, Centre Hospitalier Universitaire de Lille, Lille, France.
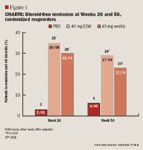
One third of patients in the adalimumab groups had complete healing of draining fistulas (Figure 2) compared with only 13% of placebo patients (P=.016).

Clinical trials with natalizumab, a humanized monoclonal antibody, had been suspended in February 2005 after 2 patients experienced progressive multifocal leukoencephalopathy (PML) while on combination therapy with natalizumab and beta interferon for the treatment of multiple sclerosis (MS).

Administration of natalizumab for the treatment of MS had been voluntarily suspended by its manufacturer in February 2005 due to the potential link to PML. FDA's Peripheral & Central Nervous System Drugs Advisory Committee met in March 2006 and recommended that natalizumab be allowed to return to the US market. In early June, FDA approved the resumption of marketing natalizumab for MS patients who have not responded adequately to or cannot tolerate other MS treatments via a special restricted distribution program.
A safety evaluation of >3,500 patients with Crohn's disease, MS, or rheumatoid arthritis who were previously treated with natalizumab in clinical trials found no additional cases of PML, said William J. Sandborn, MD, professor of medicine, Mayo Clinic College of Medicine, Rochester, Minn. The risk of PML appears to be about 1 in 1,000 with natalizumab, he determined.
Natalizumab induced a sustained response and remission in a trial of 509 patients with Crohn's disease and active inflammation, defined as a level of C-reactive protein >2.87 mg/L, said Stephen Targan, MD, Cedars-Sinai Medical Center, Los Angeles, Calif.
Significantly more patients randomized to natalizumab infusions than those randomized to placebo were therapy responders at Weeks 8 and 12 using the CDAI score as the determinant of response (48% vs 32%, respectively; P<.001). Remission was achieved by 26% of the patients in the natalizumab group versus 16% of patients in the placebo group (P=.002) at Weeks 8 and 12.
Serious adverse events occurred in 5% of the natalizumab group and 10% of the placebo group; Crohn's disease flares constituted most of the serious adverse events in the placebo group, Dr Targan said.

The study included 662 patients with moderate-to-severe Crohn's disease who were randomized to placebo or 400 mg certolizumab at Weeks 0, 2, and 4, and then every 4 weeks thereafter through Week 24.

The percentage of patients who experienced remission was superior with certolizumab versus placebo at Week 4 (19.5% vs 11.3%, respectively; P<.01). Response rates were higher in certolizumab recipients regardless of baseline CRP levels (Table 1).
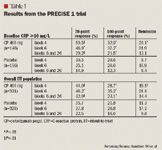
There were no opportunistic infections observed in certolizumab recipients.
"Infections are part of the package of prescribing anti-TNF drugs," said Brian G. Feagan, MD, professor of medicine, epidemiology, and biostatistics at the University of Western Ontario, London, Ontario. Opportunistic infections with anti-TNF agents have been rare.
A bigger concern is loss of efficacy because of the development of antibodies. Antibody formation seems to be greatest with infliximab-up to 61% of patients develop antibodies to infliximab after multiple exposures the medication, Dr Feagan said. "These antibodies correlate with infusion reactions and decreased duration and magnitude of treatment response," he said.
The development of antibodies with anti-TNF agents can be kept to <10% with regular maintenance infusions or injections, use of bolus IV steroids, concomitant use of immunosuppressants, and use of higher doses of anti-TNF agents, said Dr Sandborn.
In studies of certolizumab, 8% of patients had antibody formation despite few patients receiving concomitant immunosuppressants. "Most patients can get away without coadministration of immunosuppressants if certolizumab is given consistently every 4 weeks," Dr Sandborn said.
Concerns regarding lymphoma also have been raised over the anti-TNF agents, especially infliximab. In a registry of 6,273 patients being treated for Crohn's disease, patients treated with infliximab had rates of cancer or lymphoma that were no higher than rates in patients treated with other therapies for Crohn's disease, said Gary Lichtenstein, MD, associate director, Inflammatory Bowel Disease Program, University of Pennsylvania Health System, Philadelphia, Pa.
The data represent 8,314 patient-years of infliximab treatment in 3,272 patients. There were no differences in mortality or the rates of congestive heart failure between the infliximab recipients and patients treated with other Crohn's disease therapies.
In the registry, the use of corticosteroids was associated with a 2.24 hazard ratio of death compared with no corticosteroid use. Current use of steroids (prednisone) was also associated with a doubling of the risk of serious infections (P<.0001), and use of narcotic analgesics was associated with 2.72 times the risk of serious infections (P<.0001). By comparison, the hazard ratio for serious infections within 3 months of infliximab use was 1.9 (P<.001). Cox proportional hazard analysis determined that infliximab was not an independent predictor of serious infections. Both prednisone and narcotics were independently associated with serious infection upon Cox analysis.
With infliximab, "the benefit is still clearly outweighing the risk at this time," said Dr Lichtenstein. "Corticosteroids and narcotics are associated with significantly increased risk of serious infections and death." With the potential to slow disease progression and modify the disease course, early use of anti-TNF drugs has been advocated for the management of patients with Crohn's disease.
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
