- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Disease-modifying therapy in adult relapsing-remitting multiple sclerosis
Multiple sclerosis (MS) is a significant cause of disability among young adults, more commonly women, and usually strikes patients in the prime of their lives. Despite recent therapeutic advancements, MS remains an incurable, chronic illness. Clinical evidence supports the role of disease-modifying therapy early in the disease course to reduce the number of acute attacks, delay disease progression, maintain quality of life, and prevent disability.

Key Points
Abstract
Multiple sclerosis (MS) is a significant cause of disability among young adults, more commonly women, and usually strikes patients in the prime of their lives. Despite recent therapeutic advancements, MS remains an incurable, chronic illness. Clinical evidence supports the role of disease- modifying therapy (DMT) early in the disease course to reduce the number of acute attacks, delay disease progression, maintain quality of life, and prevent disability. However, an estimated 43% of patients are not receiving DMT. Current guidelines provide direction for making the diagnosis, identifying MS subtypes and choosing initial therapy for the relapsing-remitting forms of the disease. This review presents a summary of available DMT agents indicated for the treatment of patients with relapsing-remitting MS (RRMS), briefly touches upon the management of acute attacks, and highlights new therapies being investigated for RRMS, which show promise as adjuncts to or alternatives to currently accepted strategies. (Formulary. 2010;45:252–262.)
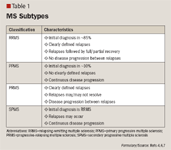
DIAGNOSIS

PATHOPHYSIOLOGY
Although the pathophysiology of MS is not completely understood, the disease is a result of damage to the myelin sheath, a protective insulating layer surrounding CNS nerve fibers.3,4,7 The myelin sheath is needed to ensure efficient and accurate impulse conduction along the axon. When this function is lost as a result of the body's immune system attacking the myelin, signal transmission becomes impaired and neurons are more vulnerable to injury. Evidence suggests that in patients with MS, T cells cross the blood-brain barrier and enter the CNS via cellular adhesion molecules (CAMs) and proinflammatory cytokines.8 Macrophages and B cells also participate in the autoimmune processes responsible for myelin degeneration. During acute attacks, inflammation and degeneration of the neuronal tissue result in multiple lesions or areas of scarring (ie, sclerosis).3,4,7 Once neuronal injury occurs, the damage is irreversible.
MONITORING PARAMETERS
In addition to identifying patients with MS and initiating effective therapy, it is important to monitor for disease progression and response to therapy. The Kurtzke Expanded Disability Status Scale (EDSS) is the most widely used instrument for monitoring disability progression.2,8 Scores range from 0 to 10, with the lowest numbers implying normal function and the highest signifying severe disability or death. MRI scans are essential in confirming the diagnosis of MS and monitoring its progression. Lesions seen on T2-weighted MRI images are nonspecific and may indicate either edema in an active lesion or a chronic inactive lesion. Lesions present upon gadolinium-enhanced scanning suggest active inflammation. Finally, lesions seen with T1-weighted, hypo-intense scans are useful indicators of tissue destruction.
MANAGING ACUTE ATTACKS
Acute MS attacks are sudden neurological disturbances persisting for at least 24 hours that result in new or augmented symptoms or disabilities.12 Symptoms typically progress over a few days, peak within a week, and then slowly resolve. It has been proposed that infection is a plausible trigger for these attacks.
Based on evidence from several class I studies and meta-analyses, current guidelines recommend high-dose corticosteroids to treat acute MS attacks. Either intravenous (IV) methylprednisolone, 500 mg to 1 g daily (the most widely used regimen) or oral methylprednisolone, 500 mg to 2 g daily, should be initiated promptly after symptom onset and continued for 3 to 5 days.5,7,13
Further studies are needed to verify whether the type, route of administration, dose, or duration of corticosteroid treatment have any impact on clinical outcomes. The mechanism responsible for the beneficial effects of corticosteroids has not been established, but limited data support immediate and short-term reduction of inflammatory edema and blood-brain barrier stabilization.14 Although corticosteroid therapy hastens recovery from acute attacks, it has not proven to slow disease progression. Short-term adverse effects include increased appetite, edema, psychosis, acne, insomnia, facial flushing, transient hyperglycemia, and hypertension. However, additional adverse effects develop with prolonged and frequent use.
The American Academy of Neurology recommends high-dose methylprednisolone for acute optic neuritis treatment. According to a small study, plasma exchange (PLEX) therapy (7 exchanges every other day) is beneficial in patients unresponsive to high-dose intravenous corticosteroids.7 Potential adverse effects of PLEX therapy include anemia, increased infection risk, fatigue, thrombosis, and hypotension. Monitoring patients with a complete blood count (CBC) prior to therapy and at regular intervals during therapy is imperative.
DISEASE-MODIFYING THERAPY
INTERFERON BETA THERAPY
IFNbs inhibit several inflammatory processes, including T-cell proliferation and tumor necrosis factor production.4 However, the exact mechanism of their therapeutic effect in MS has not been established. Adverse effects commonly associated with IFNb therapy include flu-like symptoms, local injection-site reactions, leukopenia, and liver enzyme elevation.15-17,21 IFNb therapy may exacerbate depression and should therefore be used cautiously in patients with a history of depression or other mood disorders. IFNb products requiring lower doses may be more tolerable for some patients, at the cost of reduced efficacy. In addition, interferons may trigger neutralizing antibody (NAb) development, which is more common with IFN b-1b than with IFN b-1a therapy.2,3 In clinical trials, NAbs disappeared with continued therapy; however, the long-term impact of NAbs on clinical outcomes has not been determined.2,3
IFNb-1b was the first medication that FDA approved for the treatment of MS. Its safety and efficacy were demonstrated in a multicenter, double-blind, placebo-controlled study involving 372 patients with RRMS and a history of at least 2 exacerbations in the previous year.22 Patients were randomly assigned to receive IFNb-1b 50 µg, IFNb-1b 250 µg, or placebo, administered every other day. After 2 years, exacerbation rates were significantly lower in both IFNb-1b 50 µg and 250 µg groups compared to those seen among patients receiving placebo (P<.05). A 5-year follow-up study found that patients receiving IFNb-1b 250 µg continued to exhibit less disease progression and a lower exacerbation rate compared to patients treated with placebo.23 Another 5-year study by Carmona et al supports the role of IFNb-1b 250 µg subcutaneous (SC) therapy in reducing relapse rates and disability progression, compared with untreated controls after 2 years of follow-up.24 The number of relapses within 2 years of diagnosis was associated with an early relapse following IFNb-1b therapy initiation. Furthermore, disability progression was greater among patients experiencing a relapse during the first year of therapy.24
Intramuscular IFNb-1a was approved for the treatment of MS based on results of the Multiple Sclerosis Collaborative Research Group study, a randomized, double-blind, placebo-controlled, multicenter trial in 301 patients with RRMS.25 IFNb-1a 30 µg once weekly intramuscular (IM) injection significantly delayed sustained disability progression of an increase in EDSS of at least 1 point compared to placebo (P=.02). In addition, IFNb-1a led to a significant reduction in exacerbation rate (P=.03) and MRI lesion volume and number (P<.05). Coppola et al. evaluated the long-term safety and efficacy of IFNb-1a IM 30 µg once weekly in an open-label study involving 255 patients with RRMS.26 At 3 years, 58% of patients were progression free, approximately 40% were relapse free, and 88% experienced an improvement in relapse rate from baseline. The median time to disability progression was 4.5 years.
Subcutaneous IFNb-1a was assessed in the Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis study, which compared the safety and efficacy of IFNb-1a 22 µg and 44 µg subcutaneously (SC) administered 3 times weekly versus placebo in 560 patients with RRMS.27 At 2 years, IFNb-1a SC, regardless of dose, significantly reduced the number of relapses (P<.005), delayed the time to first relapse by 3 to 5 months, and delayed disability progression, compared with placebo (P<.05). After 2 years, patients initially receiving placebo were blindly randomized to one of the two IFNb-1a SC doses. At 8 years, the continuation group exhibited fewer relapses, fewer new MRI lesions, and delayed disability progression compared to the crossover group.28 This finding reinforced the role of early therapy initiation in RRMS. A safety analysis did not identify any new adverse events. The majority of patients testing positive for antibodies developed a positive antibody titer within the first 18 months of therapy.
In the past, DMT was deferred until a patient had been diagnosed with clinically definite MS (CDMS). However, clinical studies have determined that therapy initiated at the time of presentation with a clinically isolated syndrome (CIS) may delay disease progression and decrease the frequency and severity of relapses. A meta-analysis by Melo et al pooled the results of 3 landmark trials including 1,159 patients to evaluate the role of IFNb therapy in treating patients with CIS. At 2 years, IFNb reduced the risk of conversion to CDMS with an odds ratio of 0.51 (95% CI, 0.39%–0.65%) and delayed the time to CDMS conversion by up to 363 days.29 Currently, only IFNb-1b and IFNb-1a IM are approved for the treatment of patients with CIS.15,17
While numerous studies assessing the relative safety and efficacy of the 3 available IFNb products have produced comparable results, 2 open-label, head-to-head studies suggest that IFNb administered at a lower dose or frequency may be less efficacious but better tolerated than higher dose/frequency IFNb therapy.3–6 The Independent Comparison of Interferon (INCOMIN) study directly compared IFNb-1a IM 30 µg and IFNb-1b 0.25 mg in 188 patients with RRMS.30 The primary outcome measure was the proportion of relapse-free patients. At 2 years, a significantly greater percentage of patients receiving IFNb-1b were relapse-free compared to the IFNb-1a group (51% vs 36%; P=.03). In addition, IFNb-1b therapy resulted in fewer relapses per patient (P=.03), fewer treated relapses, lower EDSS scores (2.1 vs 2.5; P=.004), and delayed sustained and confirmed disability progression (P<.001) compared to IFNb-1a therapy. Moreover, patients on IFNb-1b therapy developed fewer new MRI lesions compared to the IFNb-1a group (17% vs 34%; P<.014). The frequency of adverse events was similar between groups and tended to decline after 6 months of therapy. Injection site reactions (37% vs 8%; P<.0001) and NAbs (30% vs 7%; P=.0004) were more common in the IFNb-1b group than in patients receiving IFNb-1a after 1 year of treatment. The frequency of NAbs declined during the second year and was not correlated with negative clinical outcomes.
The Evidence of Interferon Dose-response: European North American Comparative Efficacy study was a multicenter, randomized, assessor-blind comparison of IFNb-1a 30 µg IM once weekly and IFNb-1a SC 44 µg administered 3 times weekly for up to 2 years in 677 patients.31 The primary end point was the proportion of relapse-free patients. At 44 weeks, 56% of patients receiving IFNb-1a SC remained relapse-free compared to 48% of patients randomized to the low-dose IFNb-1a IM group (P=.023). In addition, IFNb-1a SC significantly delayed the time to first relapse, decreased steroid requirements, and decreased the number of active MRI lesions compared to IFNb-1a IM therapy. NAbs did not impact clinical measures but were associated with a reduced radiologic benefit. After 48 weeks of therapy, patients initially randomized to IFNb-1a IM were allowed to switch to IFNb-1a SC and were followed for an additional 8 months.32 The crossover group experienced a significant 50% relative reduction in relapse rate (P<.001) and the number of active MRI lesions (P=.022). Adverse effects and NAbs were more frequent among patients in the higher-dose IFNb-1a SC group compared with those treated with the lower-dose IFNb-1a IM.
GLATIRAMER ACETATE
The exact mechanism by which GA exerts a positive effect in patients with MS is unknown; however, it is believed to act by inhibiting T-cell activity.18 Following administration, patients may experience a transient, systemic, immediate post-injection reaction consisting of flushing, chest pain, palpitations, anxiety, dyspnea, throat constriction, and urticaria. There are no known drug interactions with GA therapy. In addition, GA does not increase the risk of hepatotoxicity or depression, as is the case with interferon therapy.18
A meta-analysis of 3 randomized, placebo-controlled studies in a total of 540 patients showed that GA 20 mg daily administered for up to 35 months was associated with a 28% reduction in the annualized relapse rate, a 36% reduction in the total number of relapses (P<.0001), a 32% delay in the time to first relapse (322 days vs 219 days; P=.01), and a reduced risk of disability progression compared to the placebo (P=.02).33
The Rebif vs. Glatiramer Acetate in Relapsing MS Disease trial was a multicenter, parallel group, open-label study that assigned GA 20 mg once-daily or IFNb-1a 44 µg SC 3 times weekly to 764 patients with RRMS for 96 weeks.34 There was no significant difference between study groups in the primary outcome measure of time to first relapse (P=.64). In addition, the 2 groups exhibited comparable numbers of active lesions and similar disability progression from baseline. An earlier retrospective, open-label study compared GA with IFNb-1b SC 0.25 mg, IFNb-1a SC 22 µg, and IFNb-1a IM 30 µg.35 At 6 months, relapse rates were similar in all treatment groups, though relapse rate reduction at 24 months was greatest with GA therapy. Similarly, there was no significant difference between groups in EDSS scores from baseline.
MITOXANTRONE
Mitoxantrone, a synthetic anthracenedione, was originally approved in 1987 for the treatment of multiple myeloma.2,19 It is commonly used in patients with leukemias and hormone-refractory prostate cancer. In 2000, mitoxantrone was approved for the treatment of worsening RRMS in patients whose neurological function remains abnormal between relapses, at a 12 mg/m2 IV dose administered every 3 months. It is also the only drug indicated for the treatment of SPMS and is approved for patients with PRMS. Furthermore, it is the only DMT agent available generically. Mitoxantrone has several mechanisms of action.19,36 By incorporating itself into a DNA strand, mitoxantrone inhibits T-cell, B-cell, and macrophage proliferation. Moreover it decreases the secretion of pro-inflammatory cytokines and increases an anti-inflammatory response via promotion of the T-cell suppressor function. In addition, mitoxantrone inhibits macrophage-mediated myelin degradation.
Side effects frequently observed in patients receiving mitoxantrone and which can usually be easily managed include transient leukopenia and neutropenia peaking around 10 to 14 days post-infusion, liver enzyme elevation, nausea, alopecia, urinary tract infections, and bluish urine discoloration.19 However, a more serious deterrent to mitoxantrone therapy is its potential to cause cardiac toxicity. There have been reports of a significant decrease in left ventricular ejection fraction (LVEF) among MS patients receiving mitoxantrone. Moreover, myocardial damage incurred during mitoxantrone therapy may result in irreversible congestive heart failure, sometimes becoming evident only years after therapy termination. Cardiotoxicity risk increases with cumulative dosage above 100 mg/m2 .19–37 Because the maximum lifetime dose approved for patients with worsening MS is 140 mg/m2 , careful cardiac function monitoring prior to every infusion is required once the 100 mg/m2 dose threshold is exceeded. The maximum lifetime dose also limits the duration of therapy, generally to a 2-year period, a concern when treating this lifelong condition. Additional information is expected once the results of a phase IV, multicenter, 5-year observational study (RENEW), designed to assess the risk of long-term cardiotoxicity among patients receiving mitoxantrone therapy, become available.20,38
A 2005 Cochrane review assessed the role of mitoxantrone in worsening RRMS, PRMS, and SPMS.39 A total of 270 patients from 4 randomized, placebo-controlled studies were included in the analysis. At 2 years, mitoxantrone significantly reduced disability progression from baseline compared with placebo (OR: 0.3; P=.05). Additionally, patients receiving mitoxantrone therapy were less likely to have a relapse at 1 year (OR: 5.4; P=.0002) and 2 years (OR: 2.82; P=.0008) of follow up. Mitoxantrone was also associated with a reduced annualized relapse rate (P=.007) and a decrease in MRI lesions (P=.04) compared with placebo. Among mitoxantrone-treated patients, 3.6% experienced a reduction in LVEF of at least 50% (P=.11). However, none of the treated patients had developed clinically significant cardiotoxicity at 1 year after therapy.
NATALIZUMAB
Natalizumab is approved as monotherapy for the treatment of RRMS in patients who have had an inadequate response to or cannot tolerate alternative DMT.8-20 Safety and efficacy in patients with chronic progressive MS have not been established. Natalizumab is an integrin receptor antagonist. It binds to the α4 subunit of α4β 1 and α4β 7 integrins expressed on the surface of all leukocytes except neutrophils, and inhibits the adhesion of leukocytes to their counter-receptor(s). Natalizumab is believed to inhibit the interaction of the α4β 1 integrin with vascular cell adhesion molecule 1, and the α4β 7 integrin with mucosal addressin cell adhesion molecule 1, respectively. By inhibiting these interactions, natalizumab may prevent an inflammatory cascade responsible for CNS lesions.
Originally approved in 2004 for the treatment of RRMS, natalizumab was withdrawn in 2005 after 3 patients developed progressive multifocal leukoencephalopathy (PML), an opportunistic viral brain infection.3,8 FDA permitted natalizumab to again be marketed in 2006 with a black box warning cautioning about the risk of PML. Since then, 55 confirmed PML cases have been reported in association with natalizumab therapy.40 In 2008, FDA issued another warning advising of a risk of liver injury with natalizumab.3,8,20 Currently, natalizumab is available only through a restricted distribution program.
In the Natalizumab Safety and Efficacy in Relapsing Remitting Multiple Sclerosis study, 942 patients with RRMS received natalizumab 300 mg IV or placebo every 4 weeks.41 After 2 years, treatment with natalizumab led to significant 42% and 68% reductions in disability progression and clinical relapse rate, respectively, compared with placebo (P<.001). Patients receiving natalizumab therapy had 83% fewer new or enlarging lesions than patients in the placebo group (P<.001).
The Safety and Efficacy of Natalizumab in Combination with Interferon Beta-1a in Patients with Relapsing Remitting Multiple Sclerosis study evaluated treatment with combined natalizumab 300 mg IV and IFNb-1a 30 µg IM compared with IFNb-1a IM monotherapy administered for up to 116 weeks.42 Compared to IFNb-1a alone, the combination therapy resulted in a 55% reduction in annualized relapse rate (P=.001) and a 24% reduction in disability progression (P=.02). In addition, fewer new or enlarging CNS lesions were observed in the combination therapy group (P<.001). The only adverse events that were significantly more frequent in the combination group than in the IFNb-1a monotherapy group were anxiety (12% vs 8%), pharyngitis (7% vs 4%), sinus congestion (6% vs 3%), and peripheral edema (5% vs 1%). PML was identified in 2 patients treated with combination therapy.42
The phase 2 Glatiramer Acetate and Natalizumab Combination Evaluation study assessed the safety and tolerability of natalizumab when added to GA 20 mg SC versus GA therapy alone in 110 patients with RRMS treated for up to 20 weeks.43 Combination therapy resulted in lower rates of new active lesions (P=.031), new gadolinium-enhancing lesions (P=.020), and newly enlarging T2-hyperintense lesions (P=.029), compared to GA monotherapy. However, both groups exhibited comparable disability progression and relapse rates. The overall incidence of adverse events was similar between groups. Of note, natalizumab in combination with other immunomodulatory drugs is not recommended due to a potentially greater risk of PML.20 Therefore, additional long-term studies are needed to evaluate the safety of natalizumab combination therapy before such a strategy may be used in clinical practice.
DALFAMPRIDINE
Dalfampridine (previously referred to as fampridine-SR) recently received FDA approval for improving mobility and walking speed in patients with MS, a common limitation in patients with MS. It is a sustained-release formulation of 4-aminopyridine, which is thought to provide symptomatic relief by temporarily enhancing nerve signaling by blocking potassium channels on the surface of nerve fibers. In a double-blind, placebo-controlled, 14-week, phase 3 clinical trial, walking speed improved significantly in 301 patients with MS receiving oral dalfampridine 10 mg twice daily.44 The primary end point, an improvement in walking speed in at least 3 of the 4 follow-ups, was significantly improved with dalfampridine compared to placebo (34.8% vs 8.3%; P<.001). Moreover, dalfampridine increased leg strength, even in some individuals whose walking speed did not improve.
PRACTICE GUIDELINES
Three major US treatment guidelines provide guidance on RRMS pharmacotherapy. The Multiple Sclerosis Therapy Consensus Group (MSTCG) Guidelines, updated in 2008 after first being published in 2002, integrate evidence-based medicine with expert opinion to form recommendations for the use of DMT.3,45 The American Academy of Neurology guidelines published in 2002 review clinical evidence in evaluating the roles of available DMTs.6 The guidelines do not address symptomatic management of MS or novel therapeutic approaches. Finally, a consensus statement issued by the National Clinical Advisory Board of the National Multiple Sclerosis Society in 2007 establishes first-line treatment options.9
In summary, all 3 organizations agree that GA or IFNbs are appropriate first-line therapies for RRMS.5,9,45 Furthermore, due to demonstrated benefit of early treatment, DMT should be considered in patients at high risk for developing CDMS (ie, patients exhibiting evidence of CIS). The guidelines do not offer guidance regarding the selection of a particular IFNb product, while acknowledging the limited data in favor of the high dose/frequency strategy, and do not differentiate between IFNb and GA in terms of optimal initial therapy. Since failure of one first-line agent does not guarantee failure of another, patients experiencing suboptimal response with one IFNb agent may switch to another or be started on GA therapy. While the presence of NAbs has been associated with increased relapse rates, at this time NAb development does not warrant therapy change in patients responding to IFNbs. Therapy change may, however, be appropriate for suboptimal responders testing positive for NAbs. While natalizumab and mitoxantrone have demonstrated superior efficacy compared with placebo in decreasing relapses and delaying disability progression, their use is limited by their toxicity profiles. Neither drug is an appropriate first-line DMT for patients with RRMS.8,46 Natalizumab or mitoxantrone may be considered in patients who have exhausted other therapeutic alternatives or have an aggressive initial disease course.
PIPELINE DRUGS
Although the introduction of DMTs brought about considerable advancement in the treatment of RRMS, only 4 agents, the 3 INFbs and GA, are currently available for first-line use. These agents can only be administered intramuscularly or subcutaneously, which may lead to adherence issues for patients with MS, since many patients dislike self-injection or have a phobia of needles that prevents them from being able to self- inject. Mitoxantrone and natalizumab are available as second-line agents; however they are administered via IV infusion and significant toxicities limit their use. Several new investigational drugs have shown promise in MS management and may help to overcome some of the limitations of the currently available treatments.
ORAL AGENTS
The availability of oral agents for the treatment of RRMS is anxiously anticipated because oral administration could significantly impact MS therapy, especially for those patients with a fear of injections or for those who have not responded to, or who are unable to tolerate current therapies.
The first oral DMT for RRMS is likely to be the sphingosine 1-phosphate receptor modulator, fingolimod. (See Focus On article, page 245.) In June 2010, FDA's Peripheral and Central Nervous System Drugs Advisory Committee voted to recommend approval of the 0.5-mg dose of the first-in-class agent as a first-line treatment for RRMS. In a phase 3 clinical trial, 1,292 patients with RRMS and a history of at least 1 relapse were randomly assigned to receive fingolimod 1.25 mg or 0.5 mg daily or IFNb-1a 30µg IM weekly. The annual relapse rate was 0.20 (95% CI, 0.16–0.26) among those receiving fingolimod 1.25 mg, 0.16 (94% CI, 0.12–0.21) for those receiving the 0.5-mg dose, and 0.33 (95% CI, 0.26–0.42; P<.001) among patients receiving IFNb-1a.47
There was an increase in bradycardia among patients receiving their first dose of fingolimod, and treatment with the agent was associated with a risk of macular edema and a gradual decrease in pulmonary function.48 Therefore, the advisory committee recommended that cardiac monitoring be required when patients receive the first dose and that more frequent ophthalmic examinations be performed in patients continuing fingolimod treatment. In addition, the advisory committee recommended that postmarket clinical studies using a 0.25-mg dose of fingolimod be performed to determine whether the lower dose lessens adverse effects. Fingolimod is under FDA priority review with an expected approval date of September 21, 2010.
Another oral RRMS treatment candidate, cladribine, is a purine nucleoside analog currently available in an IV formulation for the treatment of hairy cell leukemia. A new drug application (NDA) for oral cladribine was resubmitted to FDA in June 2010, after the agency rejected a first NDA in November 2009, and requested additional information from the manufacturer. In July, it was granted priority review by FDA. In a phase 3 clinical trial, 1,326 patients with RRMS were randomly assigned to receive a cumulative dose of 3.5 mg/kg or 5.25 mg/kg cladribine or placebo given in 2 or 4 courses for 48 weeks followed by 2 additional short courses given at weeks 48 and 52. With this design, patients received the oral medication for a total of 8 or 20 days annually.49
The primary end point was relapse rate after 96 weeks. Researchers reported significantly lower relapse rates of 0.14 and 0.15 for the 3.5 mg/kg and 5.25 mg/kg treatment groups respectively, compared with a relapse rate of 0.33 for patients receiving placebo (P<.001). There was also a relapse-free rate of 79.7% for the 3.5 mg/kg group, 78.9% for the 5.25 mg/kg group, and 60.9% for the placebo group. Researing positive for NAbs. While natalizumab and mitoxantrone have demonstrated superior efficacy compared with placebo in decreasing relapses and delaying disability progression, their use is limited by their toxicity profiles. Neither drug is an appropriate first-line DMT for patients with RRMS.8,46 Natalizumab or mitoxantrone may be considered in patients who have exhausted other therapeutic alternatives or have an aggressive initial disease course.
PIPELINE DRUGS
Although the introduction of DMTs brought about considerable advancement in the treatment of RRMS, only 4 agents, the 3 INFbs and GA, are currently available for first-line use. These agents can only be administered intramuscularly or subcutaneously, which may lead to adherence issues for patients with MS, since many patients dislike self-injection or have a phobia of needles that prevents them from being able to self- inject. Mitoxantrone and natalizumab are available as second-line agents; however they are administered via IV infusion and significant toxicities limit their use. Several new investigational drugs have shown promise in MS management and may help to overcome some of the limitations of the currently available treatments.
ORAL AGENTS
The availability of oral agents for the treatment of RRMS is anxiously anticipated because oral administration could significantly impact MS therapy, especially for those patients with a fear of injections or for those who have not responded to, or who are unable to tolerate current therapies.
The first oral DMT for RRMS is likely to be the sphingosine 1-phosphate receptor modulator, fingolimod. (See Focus On article, page 245.) In June 2010, FDA's Peripheral and Central Nervous System Drugs Advisory Committee voted to recommend approval of the 0.5-mg dose of the first-in-class agent as a first-line treatment for RRMS. In a phase 3 clinical trial, 1,292 patients with RRMS and a history of at least 1 relapse were randomly assigned to receive fingolimod 1.25 mg or 0.5 mg daily or IFNb-1a 30µg IM weekly. The annual relapse rate was 0.20 (95% CI, 0.16–0.26) among those receiving fingolimod 1.25 mg, 0.16 (94% CI, 0.12–0.21) for those receiving the 0.5-mg dose, and 0.33 (95% CI, 0.26–0.42; P<.001) among patients receiving IFNb-1a.47
There was an increase in bradycardia among patients receiving their first dose of fingolimod, and treatment with the agent was associated with a risk of macular edema and a gradual decrease in pulmonary function.48 Therefore, the advisory committee recommended that cardiac monitoring be required when patients receive the first dose and that more frequent ophthalmic examinations be performed in patients continuing fingolimod treatment. In addition, the advisory committee recommended that postmarket clinical studies using a 0.25-mg dose of fingolimod be performed to determine whether the lower dose lessens adverse effects. Fingolimod is under FDA priority review with an expected approval date of September 21, 2010.
Another oral RRMS treatment candidate, cladribine, is a purine nucleoside analog currently available in an IV formulation for the treatment of hairy cell leukemia. A new drug application (NDA) for oral cladribine was resubmitted to FDA in June 2010, after the agency rejected a first NDA in November 2009, and requested additional information from the manufacturer. In July, it was granted priority review by FDA. In a phase 3 clinical trial, 1,326 patients with RRMS were randomly assigned to receive a cumulative dose of 3.5 mg/kg or 5.25 mg/kg cladribine or placebo given in 2 or 4 courses for 48 weeks followed by 2 additional short courses given at weeks 48 and 52. With this design, patients received the oral medication for a total of 8 or 20 days annually.49
The primary end point was relapse rate after 96 weeks. Researchers reported significantly lower relapse rates of 0.14 and 0.15 for the 3.5 mg/kg and 5.25 mg/kg treatment groups respectively, compared with a relapse rate of 0.33 for patients receiving placebo (P<.001). There was also a relapse-free rate of 79.7% for the 3.5 mg/kg group, 78.9% for the 5.25 mg/kg group, and 60.9% for the placebo group. Researchers reported a lower risk of progression of disability among those taking either the lower dose (P=.02) or the higher dose (P=.03) compared with placebo.49
Lymphocytopenia was significantly more common among patients receiving cladribine and occurred in 21.6% of patients taking the lower dose and in 31.5% of those taking the higher dose. Only 1.8% of patients receiving placebo developed lympocytopenia. Herpes zoster occurred in 8 of the patients in the low-dose cladribine group and 12 in the high-dose group and was not observed among placebo patients.49
Dimethyl fumarate (BG-12) is a component of currently available psoriasis therapies and is the first agent in trials for RRMS that activates the Nrf2 transcriptional pathway. It is being developed under an FDA Fast Track designation and is in phase 3 clinical trials for RMSS monotherapy and in phase 2 trials for use in combination with IFNbs or GA in patients with disease activity despite other monotherapy.50 The phase 2 trial includes 100 patients and 2 phase 3 trials are enrolling a total of 2,600 patients.50
In a phase 2b clinical trial of 257 patients with RRMS randomized to receive 120-mg fumarate once daily, 120 mg 3 times daily, 240 mg 3 times daily, or placebo for 24 weeks, the oral agent significantly reduced new and enlarging lesions. The 240-mg dose reduced gadolinium-enhancing lesions by 69% compared to placebo (P<.0001) and also reduced new or enlarging T2-hyperintense lesions and new T1-hyperintense lesions (P=.0006 and P=.014, respectively) compared to placebo. In addition, researchers reported that fumarate reduced the annualized relapse rate by 32% compared with placebo (P=.272). Abdominal pain, flushing, and hot flushes were more common among patients receiving active treatment, and headache, fatigue, and feeling hot were identified as dose-related adverse effects.51
Teriflunomide is a pyrimidine synthesis inhibitor with selective immunosupressive and immunomodulatory characteristics. It is the active metabolite of leflunomide, a rheumatoid arthritis therapy, and is believed to exert both antiproliferative and anti-inflammatory effects.52 Phase 3 clinical trials using teriflunomide as monotherapy in patients with RRMS are ongoing, and positive results of phase 2 trials of teriflunomide combined with IFNb or GA have recently been reported but have not been published.53 Results of a phase 2 safety and efficacy trial using teriflunomide as monotherapy demonstrated reduced lesion activity.54
Laquinimod is a second generation quinolone-3-carboxamide that showed a reduction in demyelination and induction of axonal protection in preclinical studies and some efficacy in small numbers of patients with RRMS in phase 1 and 2 clinical trials.55 Laquinimod received a Fast Track designation in February 2009, and 2 phase 3 clinical trials are under way.56
OTHER POTENTIAL AGENTS
Several monoclonal antibodies are being investigated as possible additions to the treatment armamentarium for RRMS. Although these therapies will not be orally administered, they do target different sites on immune cells and may offer alternatives for patients who have not responded to current treatment options.
Alemtuzumab was given an FDA Fast Track designation in June 2010 and 2 phase 3 trials are under way. The first compares the humanized monoclonal antibody that targets CD52 with high-dose IFNb-1a in patients with early RRMS who have no history of previous treatment. The second clinical trial compares alemtuzumab with IFNb-1a in patients showing disease activity despite receiving other therapies.57
In a phase 3 trial including 334 patients with RRMS of less than 3 years duration, alemtuzumab was superior to IFNb-1a SC in reducing the rate of sustained accumulation of disability, reducing disability score, and reducing lesion burden. Thyroid disorders, immune thrombocytopenic purpura, and infections were more common among patients receiving alemtuzumab.58 Alemtuzumab is currently approved as a treatment for B-cell chronic lymphocytic leukemia.
Rituximab, which targets and depletes CD20+ B lymphocytes, reduced lesion burden and relapse rate compared with placebo and reduced relapses when used as an add-on to conventional injectable therapies in phase 2 trials of patients with RRMS.59,60 No additional clinical trials in MS are currently under way. Rituximab is currently approved for use in non-Hodgkin's lymphoma, rheumatoid arthritis, and chronic lymphocytic leukemia.
Daclizumab appears to increase numbers of CD56bright natural killer cells and in a phase 2 clinical trial reduced by 72% the number of new or enlarging gadolinium-enhancing lesions when combined with IFNb compared to IFNb alone.61 Several additional phase 2 clinical trials in RRMS are under way and a phase 3 trial comparing daclizumab with IFNb is planned.62 Daclizumab is currently approved for prophylaxis against acute rejection in renal transplant patients.
ECONOMIC CONSIDERATIONS
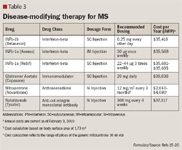
Multiple sclerosis is a lifelong debilitating condition that is associated with substantial direct and indirect healthcare costs. Patients with MS are more than twice as likely to see a healthcare professional or be hospitalized overnight as individuals without MS.63 Although the introduction of DMT for the treatment of MS has helped to reduce the frequency of acute attacks and delay disability progression, there is currently no cure. It has been estimated that relapses cost anywhere from $243 to $12,870 per episode, depending on severity.64 The bulk of the total cost of MS is composed of indirect costs (37%) incurred by formal and informal care as well as lost wages and productivity due to disability.65,66 Disease-modifying drugs, currently indicated for first-line use, are expensive and account for 34% of total direct medical costs.65 Drug acquisition cost for first-line DMT is estimated around $36,400 per patient per year (Table 3, page 256). The cost of these drugs is rising and as expert opinion continues to shift in the direction of earlier therapy initiation, utilization and hence drug expenditure are expected to increase significantly. In addition, as new pharmaceuticals make their way into the market, drug costs may rise even further.
A 2009 pharmacoeconomic study evaluated the cost-effectiveness of 4 disease-modifying drugs used for first-line treatment of MS.64 While the costs of therapy were comparable for all agents, efficacy parameters varied. IFNb-1a SC, IFNb-1b SC, and GA were associated with the least costs per relapse avoided during a 2-year period ($80,589, $87,061, and $88,310, respectively). IFNb-1a IM was found to be the most expensive option, costing $141,721 per relapse avoided. An earlier meta-analysis comparing the efficacy and costs of IFNb-1a SC and GA found that the latter was associated with the lowest probability of relapse as well as significantly lower total direct medical costs ($45,213 vs $57,311; P<.0001).1 A similar conclusion was obtained from another study which found GA to have the least incremental cost per quality-adjusted life-year ($258,465) compared to the other first-line DMT options ($303,968 to $416,301).66
Generally, the cost of MS is directly correlated with disease severity and the level of disability. Since DMT has been shown effective in reducing the number of relapses and delaying disability progression, some of the direct costs of drug acquisition are offset by their benefits. However, in consideration of the rising costs of DMT, healthcare decision-makers may consider the relative efficacy and cost of the currently available MS disease-modifying drugs in assigning formulary status.
CONCLUSION
MS is a lifelong disease that if not detected and treated early, will take a toll on patients' quality of life. Although great strides have been made in establishing the diagnosis and initiating DMT early in the disease course, the rates of relapse and disability progression are still high. Moreover, available therapies may cause adverse effects that patients cannot tolerate. In addition, the effect of NAb formation on clinical outcomes is not well understood. Current clinical guidelines concur on the importance of early therapy initiation and recommend either IFNb or GA for first-line therapy. However, there is no consensus regarding optimal management of patients who have failed first-line treatment options. More research is needed to establish effective therapy for patients with refractory RRMS, those with progressive types of MS, and for children. There are promising agents being investigated for use as DMT in RRMS patients, which may lead to expanded treatment options and improved clinical outcomes.
Dr Rosenzweig is clinical consultant pharmacist, Clinical Pharmacy Services, Commonwealth Medicine, at the University of Massachusetts Medical School. Dr Hartman is associate clinical director, Clinical Pharmacy Services, Commonwealth Medicine, University of Massachusetts Medical School. Dr MacKenzie is a transitional care clinical pharmacist, Ralph H. Johnson Veteran's Affairs Medical Center, Charleston, S.C.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
REFRENCES
1. Castelli-Haley J, Lage MJ, Oleen-Burkey MKA, Johnson KP. Glatiramer acetate versus interferon beta-1a for subcutaneous administration: comparison of outcomes among multiple sclerosis patients. Adv Ther. 2008;25(7):658–673.
2. Rizvi SA, Agius MA. Current approved options for treating patients with multiple sclerosis. Neurology. 2004;63(suppl 6):S8–S14.
3. Courtney AM, Treadaway K, Remington G, Frohman E. Multiple sclerosis. Med Clin N Am. 2009;93:451–476.
4. Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952.
5. Goodin DS, Frohman EM, Garmany GP, et al. Disease modifying therapies in multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the MS Council for Clinical Practice Guidelines. Neurology. 2002;58(2):169–178.
6. Galetta SL, Markowitz C, Lee AG. Immunomodulatory agents for the treatment of
relapsing multiple sclerosis. Arch Intern Med. 2002;62:2161–2169.
7. National Institute for Clinical Excellence (NICE). Multiple sclerosis: national clinical guideline for diagnosis and management in primary and secondary care. 2004. Available at: http://www.rcplondon.ac.uk/pubs/books/ms/. Accessed July 29, 2010.
8. Goodin DS, Cohen BA, O'Connor P, Kappos L, Stevens JC. Assessment: the use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence based review). Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2008;71:766–773.
9. National Clinical Advisory Board of the
National Multiple Sclerosis Society. MS Disease Management Consensus Statement. 2007. Modified February 2, 2010. Available at: http://www.nationalmssociety.org/download.aspx?id=8. Accessed July 29, 2010.
10. McDonald WI, Compston A, Edan G, et al. Recommended Diagnostic Criteria for Multiple Sclerosis: Guidelines from the International Panel on the Diagnosis of Multiple Sclerosis. Ann Neurol. 2001;50:121–127.
11. Polman CH, Reingold SC, Edan G, et al. Diagnostic Criteria for Multiple Sclerosis: 2005 Revisions to the "McDonald Criteria." Ann Neurol. 2005;58:840–846.
12. Leary SM, Porter B, Thompson AJ. Multiple Sclerosis: diagnosis and the management of acute relapses. Postgrad Med J. 2005; 81:302–308.
13. Sellebjerg F, Barnes D, Filippini G, et al. EFNS guideline on treatment of multiple sclerosis
relapses: report of an EFNS task force on treatment of multiple sclerosis relapses. Eur J Neurol. 2005;12 (12):939–946.
14. Martinez-Caceres EM, Barrau MA, Brieva L, Montalban X. Treatment with methylprednisolone in relapses of multiple sclerosis patients: immunological evidence of immediate and short-term but not long-lasting effects. Clin Exp Immnol. 2002;127:165–171.
15. Avonex [package insert]. Cambridge, MA: Biogen Idec, Inc; 2006.
16. Rebif [package insert]. New York, NY: Pfizer, Inc; 2008.
17. Betaseron [package insert]. Montville, NJ: Bayer Healthcare Pharmaceuticals; 2007.
18. Copaxone [package insert]. Kansas City, MO: Teva Pharmaceutical Industries, LLC; 2007.
19. Novantrone [package insert]. Rockland, MA: Serono, Inc; 2008.
20. ysabri [package insert]. Cambridge, MA: Biogen Idec, Inc; 2008.
21. National Institute for Clinical Excellence (NICE): Beta Interferon and glatiramer acetate for the treatment of multiple sclerosis. 2002. Available at: http://www.nice.org.uk/nicemedia/live/11441/32290/32290.pdf. Accessed July 29, 2010.
22. The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology.1993;43(4):655–661.
23. The UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis: final outcome of the randomized controlled trial. Neurology. 1995;45(7):1277–1285.
24. Carmona O, Sasado V, Moral E, et al. Interferon-β1b in multiple sclerosis: effect on progression of disability and clinical markers of treatment response. Eur Neurol. 2008; 60:278–284.
25. Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. Ann Neurol. 1996; 39(3):285–294.
26. Coppola G, Lanzillo R, Florio C, et al. Long-term clinical experience with weekly interferon beta-1a in relapsing multiple sclerosis. Eur J Neurol. 2006;13:1014–1021.
27. PRISMS Study Group. Randomized double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet.1998;352:1498–1504.
28. Kappos L, Traboulsee A, Constantinescu C, et al. Long-term subcutaneous interferon beta-1a therapy in patients with relapsing-remitting MS. Neurology. 2006;67:944–953.
29. Melo A, Rodrigues B, Bar-Or A. Beta interferons in clinically isolated syndromes. Arq Neuropsiquiatr.2008;66(1):8–10.
30. Durelli L, Verdun E, Barbero P, et al. Every-other-day interferon beta-1b versus once weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomized multicenter study (INCOMIN). Lancet. 2002;359:1453–1460.
31. Panitch H, Goodin D, Francis G, et al. Benefits of high-dose, high-frequency interferon beta-1a in relapsing-remitting multiple sclerosis are sustained to 16 months: final comparative results of the EVIDENCE trial. J Neurol Sci. 2005;239:67–74.
32. Traboulsee A, Sabbagh AL, Bennett R, et al. Reduction in magnetic resonance imaging T2 burden of disease in patients with relapsing-remitting sclerosis: analysis of 48-week data from the EVIDENCE (evidence of interferon dose-response: European North American comparative efficacy) study. BMC Neurol. 2008;8:11.
33. Boneschi F, Rovaris M, Johnson KP, et al. Effects of glatiramer acetate on relapse rate and accumulated disability in multiple sclerosis: meta-analysis of three double-blind, randomized, placebo-controlled clinical trials. Mult Scler. 2003;9:349–355.
34. Mikol DD, Barkhof F, Chang P, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the Rebif vs Glatiramer Acetate in Relapsing MS Disease [REGARD Study). Lancet Neurol. 2008;7:903–914.
35. Haas J, Firzlaff M. Twenty-four-month comparison of immunomodulatory treatments–a retrospective open label study in 308 RRMS patients treated with beta interferons or glatiramer acetate (Copaxone). Eur J Neurol. 2005;12:425–431.
36. Fox EJ. Mechanism of action of mitoxantrone. Neurology. 2004;63(suppl 6):S15–S18.
37. Cohen BA, Mikol DD. Mitoxantrone treatment of multiple sclerosis: safety considerations. Neurology. 2004;63(suppl 6):S28–S32.
38. National Institutes of Health. http://ClinicalTrials.gov. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00262314?term=renew&rank=2. Accessed July 27, 2010.
39. Martinelli BF, Rovaris M, Capra R, et al. Mitoxantrone for multiple sclerosis. Cochrane Database of Systematic Reviews. 2005; Issue 4. Art.No.:CD002127.DOI:10.1002/14651858.CD002127.pub2.
40. Thomson Reuters. Biogen reports six new PML cases with Tysabri: correction. Reuters. Available at: http://www.reuters.com/article/idUSN1725307720100617. Updated June 17, 2010. Accessed July 29, 2010.
41. Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910.
42. Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. NEngl J Med. 2006;354:911–923.
43. Goodman AD, Rossman H, Bar-Or A, et al. GLANCE: results of a phase 2, randomized, double-blind, placebo-controlled study.
Neurology. 2009;72:806–812.
44. Goodman AD, Brown TR, Krupp LB. Fampridine MS-F203 Study Group. Sustained-release oral fampridine in multiple sclerosis: a randomized, double-blind, controlled trial. Lancet. 2009;373:732–738.
45. Multiple Sclerosis Therapy Consensus Group (MSTCG). Basic and escalating immunomodulatory treatments in multiple sclerosis: current therapeutic recommendations. J Neurol. 2008;255:1449–1463.
46. Goodin DS, Arnason BG, Coyle PK, et al. The use of mitoxantrone (Novantrone) for the treatment of multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2003;61:1332–1338.
47. Cohen JA, Barkhof F, Comi G, The TRANSFORMS Study Group. Oral Fingolimod or Intramuscular Interferon for Relapsing Multiple Sclerosis. N Engl J Med. 2010;362:P402–415.
48. Food and Drug Administraion Peripheral and Central Nervous System Drugs Advisory Committee. Briefing Materials. June 10, 2010. Available at: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PeripheralandCentralNervousSystemDrugsAdvisoryCommittee/ucm216547.htm. Accessed July 29, 2010.
49. Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362:416–426.
50. National Institutes of Health. http://ClinicalTrials.gov. Available at: http://clinicaltrials.gov/ct2/results?term=fumarate&recr=&rslt=&type=&cond=multiple+sclerosis&intr=&outc=&lead=&spons=&id=&state1=&cntry1=&state2=&cntry2=&state3=&cntry3=&locn=&gndr=&rcv_s=&rcv_e=&lup_s=&lup_e=. Accessed July 29, 2010.
51. Kappos L, Gold R, Miller DH, et al. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: a multicentre, randomized, double-blind, placebo-controlled phase IIb study. Lancet. 2008;372:1463–1472.
52. Tallantyre E, Evangelou N, Constantinescue CS. Spotlight on teriflunomide. Intl MS J. 2008;15:62–68.
53. National Institutes of Health. http://ClinicalTrials.gov. Available at: http://clinicaltrials.gov/ct2/results?term=teriflunomide+MS. Accessed July 29, 2010.
54. O'Connor PW, Li D, Freedman MS, et al. A phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology. 2006;66:894–900.
55. Tselis A. Laquinimod, a new oral autoimmune modulator for the treatment of relapsing-remitting multiple sclerosis. Curr Opin Investig Drugs. 2010;11:577–585.
56. National Institutes of Health,. http://ClinicalTrials.gov. Available at: http://clinicaltrials.gov/ct2/results?term=Laquinimod&recr=&rslt=&type=&cond=multiple+sclerosis&intr=&outc=&lead=&spons=&id=&state1=&cntry1=&state2=&cntry2=&state3=&cntry3=&locn=&gndr=&rcv_s=&rcv_e=&lup_s=&lup_e=. Accessed July 29, 2010
57. National Institutes of Health. http://ClinicalTrials.gov. Available at: http://clinicaltrials.gov/ct2/results?term=alemtuzumab+MS. Accessed July 29, 2010.
58. The CAMMS223 Trial Investigators. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med. 2008;359:1786–1801.
59. Hauser SI, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688.
60. Nalsmith RT, Piccio L, Lyons JA, et al. Rituximab add-on therapy for breakthrough relapsing multiple sclerosis: a 52-week phase II trial. Neurology. 2010;74:1860–1867.
61. Wynn D, Kaufman M, Montalban X, et al. Daclizumab in active relapsing multiple sclerosis (CHOICE study): a phase 2, randomized, double-blind, placebo-controlled, add-on trial with interferon beta. Lancet Neurology. 2010;9:381–390.
62. National Institutes of Health. http://ClinicalTrials.gov. Available at: http://clinicaltrials.gov/ct2/results?term=daclizumab+multiple+sclerosis. Accessed July 29, 2010.
63. Naci H, Fleurence R, Birt J, Duhig A. Economic burden of multiple sclerosis: a systematic review of literature. Pharmacoeconomics. 2010;28(5):363–379.
64. Goldberg LD, Edwards NC, Fincher C, et al. Comparing the cost-effectiveness of disease-modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. J Manag Care Pharm. 2009;15:543–555.
65. Kobelt G, Berg J, Atherly D, et al. Costs and quality of life in multiple sclerosis: a cross-sectional study in the United States. Neurology. 2006;66:1696–1702.
66. Bell C, Graham J, Earnshaw S, et al. Cost-effectiveness of four immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on long-term clinical data. J Manag Care Pharm. 2007;13:245–261.
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
