- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
DPP-IV inhibitors: A review of sitagliptin, vildagliptin, alogliptin, and saxagliptin
Treatment of diabetes with DPP-IV inhibitors; sitagliptin, vildagliptin, alogliptin, and saxagliptin.

Key Points

Earlier and more aggressive therapy is needed to achieve better control of diabetes. The American Diabetes Association (ADA) guidelines state that metformin, along with lifestyle changes, should be considered first-line therapy in patients with type 2 diabetes. If diabetes remains uncontrolled with first-line therapy or if contraindications to metformin therapy exist, step 2 therapies, including insulin, sulfonylureas, or thiazolidinediones (TZDs), may be employed.3 The use of these traditional agents may be limited, however, because of several factors. Some medications, such as sulfonylureas, can lose their effectiveness over time.2 Recent evidence regarding rosiglitazone, a TZD, indicates that this agent may increase the risk of cardiovascular disease.4 Although metformin and TZDs treat insulin resistance, they do not address the progressive decline in beta-cell function observed in patients with type 2 diabetes.
As a result, new avenues of treatment are required. One approach is to target the incretin mimetic hormones, a key area of previously unexplored pathophysiology. Incretin hormones are secreted by intestinal cells in response to a meal, provoking glucose-induced pancreatic insulin secretion.5
This article focuses on 4 DPP-IV inhibitors: sitagliptin, vildagliptin, alogliptin, and saxagliptin. Sitagliptin was approved by FDA in 2006. Vildagliptin was deemed approvable by FDA in February 2007; in the approvable letter, FDA requested additional data regarding the safety and efficacy of vildagliptin in renally impaired patients.7 An NDA was submitted for alogliptin in January 2008. Saxagliptin is currently being evaluated in phase 3 clinical trials.
PHARMACOLOGY
To understand the mechanism of action associated with the gliptin class, one must understand the function of the incretin hormones. An incretin hormone is defined by the following criteria: released from the gut in response to nutrients, stimulates insulin release in a concentration easily achieved after meal ingestion, and causes glucose-dependent insulin release.8 One hormone meeting these criteria is GLP-1. GLP-1 is known to exert many beneficial effects; however, these beneficial effects are quickly terminated because of the breakdown of GLP-1 by the DPP-IV enzyme.9 DPP-IV is found throughout the body, however, the highest concentrations are found in the kidneys, intestines, and bone marrow.5,6
A clinical trial comparing 12 patients with type 2 diabetes with 12 matched healthy volunteers was conducted to examine whether patients with diabetes have altered concentrations of intact GLP-1.10 Results of this study demonstrated that at 75, 90, and 120 minutes after a meal, patients with diabetes had significantly lower concentrations of GLP-1. Researchers also observed that insulin and C-peptide levels were decreased overall among patients with diabetes. Based on these results, researchers have suggested that inhibition of the DPP-IV enzyme could help compensate for the reduced levels of GLP-1 found in patients with diabetes by allowing the beneficial effects of GLP-1 to be prolonged.
SITAGLIPTIN
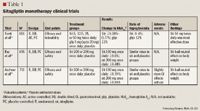

In another trial evaluating sitagliptin as monotherapy, 521 patients (mean baseline HbA1c, 8.1%) were randomized to receive sitagliptin 100 or 200 mg once daily or placebo for 18 weeks.12 The results demonstrated a significant decrease in HbA1c of 0.6% and 0.48% in patients taking sitagliptin 100 or 200 mg, respectively, versus placebo (P<.001). In addition, the incidence of hypoglycemia with sitagliptin treatment was similar to the incidence with placebo treatment; sitagliptin was also demonstrated to be weight neutral.
Aschner et al13 randomized 741 patients (baseline HbA1c, 8.0%) to monotherapy with sitagliptin 100 or 200 mg once daily or placebo for 24 weeks. The investigators observed that sitagliptin 100 or 200 mg significantly reduced HbA1c by 0.79% and 0.94%, respectively, versus placebo (P<.001). Sitagliptin also had a neutral effect on body weight. The incidence of hypoglycemia was similar among the groups. However, investigators observed that sitagliptin was associated with slightly more gastrointestinal (GI) disturbances than placebo.
Combination therapy. A small randomized, double-blind, placebo-controlled, crossover study in 28 patients was conducted to assess the effect of adding sitagliptin 50 mg twice daily or placebo to metformin ≥1,500 mg/d.14 Baseline HbA1c values ranged from 6.5% to 9.6%, and baseline mean fasting plasma glucose (FPG) was 152 mg/dL. After receiving sitagliptin or placebo for 4 weeks, patients then received the opposite treatment for 4 weeks. The results demonstrated that adding sitagliptin to ongoing metformin therapy reduced FPG by 20.3 mg/dL versus placebo (P<.05). In addition, the investigators observed that adding sitagliptin to metformin therapy had no effect on GI complaints, hypoglycemia, or changes in weight. Two larger trials (N=1,056 and N=701) also examined the effect of adding sitagliptin to ongoing metformin therapy.15,16 In these trials, the addition of sitagliptin 100 mg to metformin therapy resulted in a 0.65% to 2.07% decrease in HbA1c. The incidence of hypoglycemia in all groups was similar to the incidence reported in patients taking placebo. In addition, sitagliptin produced little to no effect on body weight in either trial.
Nauck et al17 conducted a noninferiority trial to determine whether adding sitagliptin to metformin was as effective as adding glipizide to metformin. This was a randomized, parallel-group study with an active-controlled, double-blind treatment period. A total of 1,172 patients (mean baseline HbA1c, 7.5%) were randomized to receive sitagliptin 100 mg once daily or glipizide 5 mg (up to 20 mg) once daily in addition to metformin ≥1,500 mg/d for 52 weeks. Both treatment regimens resulted in a decrease in HbA1c of 0.67%, thus confirming noninferiority. Sitagliptin therapy resulted in a significantly lower incidence of hypoglycemia versus glipizide (P<.001). Sitagliptin was also associated with significant weight loss versus glipizide (sitagliptin, –1.5 kg; glipizide, +1.1 kg; between-group difference, –2.5 kg; P<.001).
A randomized, double-blind, placebo-controlled, parallel-group study lasting 24 weeks examined the effect of adding sitagliptin to treatment with glimepiride alone or in combination with metformin.18 A total of 441 patients (mean HbA1c, 8.3%) were randomized to receive sitagliptin 100 mg once daily or placebo in addition to glimepiride ≥4 mg once daily alone or in combination with metformin ≥1,500 mg/d. After 24 weeks, the investigators observed that the addition of sitagliptin decreased HbA1c by 0.74% (P<.001) and FPG by 20.1 mg/dL (P<.001). In this trial, the addition of sitagliptin resulted in a greater incidence of hypoglycemia (P<.001) and significant weight gain (P<.001) versus placebo.
One study assessed the effect of adding sitagliptin to the treatment regimen of patients whose diabetes was uncontrolled with pioglitazone therapy.19 In this randomized, double-blind, placebo-controlled, parallel-group study, 353 patients (mean baseline HbA1c, 8.1%) received sitagliptin 100 mg once daily or placebo in addition to pioglitazone 30 or 45 mg/d for 24 weeks. The addition of sitagliptin resulted in a decrease in HbA1c of 0.7% (P<.001) and a decrease in FPG of 17.7 mg/dL (P<.001). The incidence of hypoglycemia was similar between groups; however, patients in the sitagliptin group experienced significantly more abdominal pain than patients in the placebo group. In addition, changes in body weight were similar between the sitagliptin and placebo groups.
VILDAGLIPTIN

A 12-week study by Ristic et al21 evaluated the effect of vildagliptin versus placebo in 279 patients with a baseline HbA1c of 7.7%. Patients were treated with vildagliptin 25 mg twice daily or 25, 50, or 100 mg once daily or placebo. At study end, patients who were treated with vildagliptin 50 mg once daily experienced a 0.43% decrease in HbA1c versus placebo (P=.003), and patients treated with vildagliptin 100 mg once daily experienced a 0.40% decrease in HbA1c versus placebo (P=.004). A significant decrease in PPG levels was observed in patients treated with vildagliptin 50 mg once daily versus placebo (P=.012). Although a decrease in PPG levels was also noted in patients treated with vildagliptin 100 mg once daily versus placebo, this difference did not reach statistical significance. Hypoglycemic event rates were similar among all groups, including placebo, and rates were not dose related. Two cases of hypoglycemia were considered symptomatic, both of which occurred in patients taking vildagliptin. Treatment had a neutral effect on body weight throughout the trial.
In another 12-week study, the efficacy of vildagliptin versus placebo in medication-naïve patients was evaluated as the primary end point.22 Patients (mean baseline HbA1c, 8.0%) were randomized to receive vildagliptin 25 mg twice daily (n=70) or placebo (n=28). In patients treated with vildagliptin, both FPG and PPG levels were significantly reduced versus placebo (FPG, P=.0043; PPG, P<.001). HbA1c was also reduced by 0.6%±0.2% with vildagliptin versus placebo (P=.0012). One episode of hypoglycemia occurred in the vildagliptin treatment arm; the study authors attributed this episode to a delayed meal. The between-group difference in adjusted mean change from baseline to end of study in body weight was 0.5 kg; this difference was not statistically significant.
Dejager et al23 conducted a 24-week, double-blind, randomized, multicenter, placebo-controlled, parallel-group study in 632 drug-naïve patients (mean baseline HbA1c, 8.4%) with type 2 diabetes. Patients were randomized to receive vildagliptin 50 mg twice daily, 50 or 100 mg once daily, or placebo. In all vildagliptin treatment groups, HbA1c was reduced significantly versus placebo. In patients treated with vildagliptin 50 mg twice daily, HbA1c was reduced by 0.7%±0.1% (P<.001); in patients treated with vildagliptin 50 mg once daily, HbA1c was decreased by 0.8%±0.2% (P=.003); and in patients treated with vildagliptin 100 mg once daily, HbA1c was reduced by 0.9%±0.1% (P<.001). However, in patients with the highest baseline HbA1c (8.8%–9.0%), there was a greater reduction in HbA1c among patients treated with vildagliptin 50 mg twice daily or 100 mg once daily compared with patients treated with 50 mg once daily. Body weight was decreased from baseline in all groups (–1.8 kg, –0.3 kg, –0.8 kg, and –1.4 kg with vildagliptin 50 mg once daily, 50 mg twice daily, 100 mg once daily, and placebo, respectively). Two patients who received vildagliptin 50 mg once daily and 1 patient who received vildagliptin 100 mg once daily reported hypoglycemia. Another 24-week study that used the same vildagliptin treatment regimens demonstrated similar results.24 Patients (N=340; mean baseline HbA1c, 8.4%) treated with vildagliptin 50 mg once daily experienced a decrease in HbA1c of 0.5%±0.2% versus placebo (P=.011); those treated with vildagliptin 50 mg twice daily experienced a decrease of 0.7%±0.2% versus placebo (P<.001), and those treated with vildagliptin 100 mg once daily experienced a decrease of 0.9%±0.2% versus placebo (P<.001). Body weight did not change significantly in any treatment arm. No confirmed hypoglycemia occurred in any treatment group. As with the previous studies mentioned, the authors concluded that vildagliptin produces meaningful decreases in blood sugar, is well tolerated, and is not associated with weight gain.
Another study compared the efficacy and tolerability of vildagliptin with the efficacy and tolerability of rosiglitazone in a 24-week, double-blind, randomized trial.25 Patients (N=786; baseline HbA1c, 8.7%) received either vildagliptin 100 mg daily or rosiglitazone 8 mg once daily. HbA1c levels decreased from baseline to a similar extent in the 2 groups. Vildagliptin decreased HbA1c by 1.1%±0.1% (P<.001), and rosiglitazone decreased HbA1c by 1.3%±0.1% (P<.001). These results demonstrated vildagliptin's noninferiority versus rosiglitazone. Patients in the rosiglitazone group were more likely to experience edema and increased body weight; these effects were not observed among patients in the vildagliptin group. The authors concluded that vildagliptin was as effective as rosiglitazone in the treatment of patients with type 2 diabetes, with the added benefit of not causing weight gain. One patient in each group experienced 1 mild hypoglycemic episode; no serious hypoglycemic events were reported.
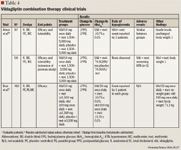
Combination therapy. In a clinical trial by Ahren et al,26 patients (mean baseline HbA1c, 7.7%±0.1%) currently taking metformin 1,500 to 3,000 mg/d were randomized to also receive treatment with vildagliptin 50 mg once daily (n=56) or placebo (n=51) for 12 weeks. The primary end point was change in HbA1c from baseline. No change in HbA1c was noted in the placebo group; however, a mean decrease of 0.7%±0.1% was noted in the combination group versus placebo (P<.0001). A 40-week, randomized, double-blind, placebo-controlled extension trial was then conducted.26 At Week 52, HbA1c had increased in both the vildagliptin plus metformin group (0.0128% per month) and the placebo plus metformin group (0.066% per month). The increase in HbA1c was more pronounced in the placebo group, suggesting that the combination of vildagliptin and metformin may slow the rate of progressive deterioration in glycemic control. The difference in HbA1c between the groups remained statistically significant throughout the trial (P=.0243). The between-group difference in HbA1c was –1.1%±–0.2% at study end (P<.0001). No hypoglycemic episodes or significant changes in weight relative to placebo were observed in the extension trial.
A larger study by Bosi et al27 also evaluated vildagliptin in combination with metformin. Patients (mean baseline HbA1c, 8.4%±1.0%) were randomized to receive vildagliptin 50 mg once daily (n=177), vildagliptin 100 mg once daily (n=185), or placebo (n=182) in addition to metformin ≥1,500 mg/d for 24 weeks. Patients treated with vildagliptin 50 mg plus metformin demonstrated a reduction in HbA1c of 0.7%±0.1% versus placebo (P<.001), and patients treated with vildagliptin 100 mg plus metformin demonstrated a reduction of 1.1%±0.1% versus placebo (P<.001). The authors concluded that vildagliptin produced meaningful decreases in HbA1c in patients whose diabetes was inadequately controlled with metformin. Body weight was unchanged relative to placebo in patients treated with vildagliptin 50 mg, but patients treated with vildagliptin 100 mg demonstrated an increase of 1.2 kg±0.4 kg relative to placebo. One patient in each group experienced a mild hypoglycemic event.
Vildagliptin was evaluated in combination with pioglitazone in a 6-month, randomized study of 592 treatment-naïve patients.28 These results were presented at the 2006 ADA 66th Annual Scientific Sessions. Patients (mean baseline HbA1c, 8.7%) were treated with pioglitazone 30 mg once daily, vildagliptin 100 mg once daily, vildagliptin 100 mg once daily plus pioglitazone 30 mg once daily, or vildagliptin 50 mg once daily plus pioglitazone 15 mg once daily. Vildagliptin 100 mg once daily plus pioglitazone 30 mg once daily resulted in a statistically significant reduction in HbA1c levels compared with patients assigned to pioglitazone alone (1.9% vs 1.4%; P<.001). Although this abstract supports the efficacy of this combination, this information is limited by the inability to fully assess study quality and design.
Another study presented at the 2006 ADA 66th Annual Scientific Sessions randomized 256 patients (mean baseline HbA1c, 8.9%) currently taking insulin (mean daily dose, 82 units) to vildagliptin 50 mg twice daily or placebo in addition to their insulin therapy.29 After 24 weeks, HbA1c had decreased by 0.5%±0.1% in the vildagliptin group compared with 0.2%±0.1% in the placebo group (P=.022). In a subgroup analysis, patients aged ≥65 years who were treated with vildagliptin plus insulin experienced a 0.7%±0.1% decrease in HbA1c versus no change in patients treated with placebo (P<.001). The incidence of side effects was similar between the groups. Hypoglycemia was less common in the vildagliptin group (vildagliptin, 113 events; placebo, 185 events); this may reflect improved glucose sensitivity with vildagliptin. No changes in body weight were reported.
ALOGLIPTIN
Available trial data for alogliptin are currently limited to abstract form. One double-blind, placebo-controlled, parallel-group, multidose study was conducted to determine the pharmacokinetics, pharmacodynamics, efficacy, and tolerability of multiple doses of alogliptin.30 A total of 55 patients who were either newly diagnosed or who had not received diabetic therapy for the previous 3 months were randomized to receive alogliptin 25 mg (n=15), 100 mg (n=14), or 400 mg (n=15) or placebo (n=11) once daily for 14 days. The area under the curve (AUC) and maximum plasma concentration (Cmax) increased in a dose-proportionate manner from Day 1 to Day 14. The time to Cmax (tmax) was demonstrated to be 1 hour. The half-life of alogliptin ranged from 12 to 21 hours on Day 14. Peak DPP-IV inhibition ranged from 93% to 98% on Days 1 and 14, with a median time to peak inhibition of 1 to 2.5 hours. On Day 14, mean 4-hour plasma glucose levels were decreased significantly from baseline in the alogliptin group versus the placebo group after each of 3 meals (P<.05). The study investigators observed that alogliptin was well tolerated, with no serious adverse events reported. The incidence of hypoglycemia was similar among groups.
SAXAGLIPTIN
Saxagliptin is currently in phase 3 trials; data are limited to abstract form. In a randomized, placebo-controlled study, 743 patients (mean baseline HbA1c, 8.0%±0.9%) with diabetes that was uncontrolled with metformin received saxagliptin 2.5, 5, or 10 mg once daily or placebo in addition to metformin 1,500 to 2,550 mg/d.31 After 24 weeks, patients treated with saxagliptin 2.5, 5, or 10 mg once daily plus metformin demonstrated significant reductions in HbA1c compared with patients who received placebo (0.73%, 0.83%, and 0.71%, respectively; P<.0001). FPG was also significantly reduced in patients receiving saxagliptin 2.5, 5, or 10 mg daily plus metformin versus those patients treated with placebo (16 mg/dL, 24 mg/dL, and 21 mg/dL, respectively; P<.0001). Saxagliptin was also demonstrated to decrease postprandial glucagon and increase postprandial insulin and C-peptide levels after oral glucose tolerance tests versus placebo. No increase in hypoglycemia was observed with saxagliptin as compared with placebo. Weight changes were similar for all patients (–1.5, –0.9, –0.5, and –1.0 kg for saxagliptin 2.5, 5, and 10 mg and placebo, respectively). Ongoing clinical trials evaluating the use of saxagliptin as monotherapy and as part of combination therapy should provide greater insight into the appropriate dosing of this agent as well as its safety and efficacy profile.
DOSING, ADMINISTRATION, AND COST
Vildagliptin, alogliptin, and saxagliptin are currently investigational. Therefore, formal recommendations for cost and dosing are not yet available. Based on the numerous available studies for vildagliptin, an oral dose of 50 or 100 mg once daily appears to be effective. There are only limited data available for alogliptin and saxagliptin at this time; the exact dosage form, strength, and dosing interval are yet to be determined.
Sitagliptin is currently available in 25-mg, 50-mg, and 100-mg tablets. The recommended dose is 100 mg once daily as monotherapy or in combination with metformin or a peroxisome proliferator-activated receptor gamma (PPAR-gamma) agonist (eg, TZDs). Patients with a creatinine clearance of 30 to 50 mL/min should be treated with 50 mg once daily; patients with a creatinine clearance <30 mL/min should be treated with 25 mg once daily.32 The average wholesale price (AWP) for a 1-month supply of the 100-mg tablets is approximately $174.96.33
ADVERSE EVENTS
In clinical studies, DPP-IV inhibitors have generally been well tolerated. Reported events are often mild and include nasopharyngitis, upper respiratory tract infection, headache, and cough.20,21,26,29 Clinical trials have indicated that DPP-IV inhibitors generally do not cause severe hypoglycemia or weight gain. This is likely secondary to the mechanism of action of these agents; insulin secretion is stimulated in a dose-dependent manner, thereby minimizing hypoglycemia and resulting weight gain. The weight neutrality of this class distinguishes these agents from other commonly used antidiabetic medications, including insulin, sulfonylureas, and TZDs.
DPP-IV has the potential to cleave other substrates besides GLP-1.34 These substrates include hormones, neuropeptides, and chemokines. In many cases, this cleavage product is inactive. DPP-IV inhibitors prolong the action of neuropeptides such as substance P and macrophage-derived chemokines. Prolonging these messengers may produce inflammation (effect on substance P), increase blood pressure (effect on neuropeptide Y), or cause allergic reactions (effect on chemokines). These theoretical concerns have not yet been observed in animal or human studies.
DRUG INTERACTIONS
Limited data are available regarding drug interactions associated with the use of vildagliptin, alogliptin, and saxagliptin. Clinical studies to date indicate no documented drug interactions with these agents. Both vildagliptin and sitagliptin have been studied in combination with other antidiabetic agents and have not been demonstrated to alter the efficacy of these antidiabetic agents. When digoxin has been used concurrently with sitagliptin 100 mg once daily, a slight increase in both the AUC (11%) and Cmax (18%) of digoxin has been observed.32 Patients being treated with digoxin and sitagliptin concurrently should therefore be monitored.
CONCLUSION
Sitagliptin and vildagliptin have been demonstrated to significantly reduce HbA1c when used as monotherapy. However, these reductions are typically not as significant as those achieved with more traditional therapies. Furthermore, monotherapy studies published to date have included patients with relatively mild elevations in HbA1c levels at baseline. Thus, there are few data on the efficacy of these agents as monotherapy in patients with poorly controlled disease. Trials have demonstrated that DPP-IV inhibitors in combination with traditional agents are associated with significant decreases in HbA1c and FPG levels. Advantages of these agents include the ability to achieve sustainable reductions in HbA1c, few adverse events, neutral effect on body weight, and a once-daily oral dosing regimen. In addition, this class may preserve or even reverse the decline in beta-cell function that is observed in patients with diabetes. Further long-term data are required to support the use of these agents early in treatment. Potential disadvantages include cost and the relative lack of long-term safety and efficacy data. Whether these agents have any effect on cardiovascular disease is also currently unknown.
Metformin is generally considered first-line therapy for patients with type 2 diabetes. However, many patients will require additional therapy to achieve therapeutic goals. Sulfonylureas, TZDs, insulin, DPP-IV inhibitors, and other incretin mimetics are viable options for patients whose diabetes is uncontrolled with metformin. Should long-term data further support the effect of DPP-IV inhibitors on morbidity and mortality, these agents could be considered first-line therapy in the future.
Dr Miller is an adjunct clinical assistant professor, University of Florida, Orlando campus, and a faculty member for a family practice residency, Florida Hospital East, Orlando. Dr St. Onge is campus director and a clinical assistant professor, University of Florida College of Pharmacy, Orlando campus. Dr Taylor is a clinical associate professor, University of Florida Department of Pharmacy Practice, Gainesville.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
REFERENCES
1. Centers for Disease Control and Prevention website. Diabetes: Sounding the alert on a debilitating disease. http://www.cdc.gov/features/diabetesalert/. Accessed March 21, 2008.
2. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: Progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–2012.
3. Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes [erratum in Diabetes Care. 2006;49:2816–2818]. Diabetes Care. 2006;29:1963–1972.
4. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes [erratum in N Engl J Med. 2007;357:100]. N Engl J Med. 2007;356:2457–2471.
5. McIntosh CH, Demuth HU, Pospisilik JA, Pederson R. Dipeptidyl peptidase IV inhibitors: How do they work as new antidiabetic agents? Regul Pept. 2005;128:159–165.
6. Weber AE. Dipeptidyl peptidase IV inhibitors for the treatment of diabetes. J Med Chem. 2004;47:4135–4141.
7. Lauster CD, McKaveney TP, Muench SV. Vildagliptin: A novel oral therapy for type 2 diabetes mellitus. Am J Health Syst Pharm. 2007;64:1265–1273.
8. Creutzfeldt W. The [pre-] history of the incretin concept. Regul Pept. 2005;128:87–91.
9. Kim D, Wang L, Beconi M, et al. (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: A potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem. 2005;48:141–151.
10. VilsbØll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609–613.
11. Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract. 2007;61:171–180.
12. Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H; Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564–2571.
13. Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE; Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–2637.
14. Brazg R, Xu L, Dalla Man C, Cobelli C, Thomas K, Stein PP. Effect of adding sitagliptin, a dipeptidyl peptidase-4 inhibitor, to metformin on 24-h glycaemic control and beta-cell function in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:186–193.
15. Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE; Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979–1987.
16. Charbonnel B, Karasik A, Liu J, Wu M, Meininger G; Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638–2643.
17. Nauck M, Meininger G, Sheng D, Terranella L, Stein PP; Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: A randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007;9:194–205.
18. Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P; Sitagliptin Study 035 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007; 9:733–745.
19. Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P; Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: A 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556–1568.
20. Ahren B, Landin-Olsson M, Jansson PA, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:2078–2084.
21. Ristic S, Byiers S, Foley J, Holmes D. Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: Vildagliptin (LAF237) dose response. Diabetes Obes Metab. 20057:692–698.
22. Pratley RE, Jauffret-Kamel S, Galbreath E, Holmes D. Twelve-week monotherapy with the DPP-4 inhibitor vildagliptin improves glycemic control in subjects with type 2 diabetes. Horm Metab Res. 2006;38:423–428.
23. Dejager S, Razac S, Foley JE, Schweizer A. Vildagliptin in drug-naïve patients with type 2 diabetes: A 24-week, double-blind, randomized, placebo-controlled, multiple-dose study. Horm Metab Res. 2007;39:218–223.
24. Pi-Sunyer FX, Schweizer A, Mills D, Dejager S. Efficacy and tolerability of vildagliptin monotherapy in drug-naïve patients with type 2 diabetes. Diabetes Res Clin Pract. 2007;76:132–138.
25. Rosenstock J, Baron MA, Dejager S, Mills D, Schweizer A. Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes : A 24-week, double-blind, randomized, trial [erratum in Diabetes Care. 2007;30:1330]. Diabetes Care, 2007;30:217–223.
26. Ahren B, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase IV inhibitor LAF237 in metformin-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2874–2880.
27. Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890–895.
28. Rosenstock J, Baron MA, Lebeaut A. The use of vildagliptin for treatment of patients with type 2 diabetes [abstract]. Presented at: American Diabetes Association 66th Annual Scientific Sessions; June 9–13, 2006; Washington, DC. Abstract 6-LBCS.
29. Fonseca V, Dejager S, Albrecht D, Shirt L, Schweizer A. Vildagliptin as add-on to insulin in patients with type 2 diabetes (T2DM) [abstract]. Presented at: American Diabetes Association 66th Annual Scientific Sessions; June 9–13, 2006; Washington, DC. Abstract 467-P.
30. Christopher R, Covington P, Davenport M, et al. Pharmacokinetics, pharmacodynamics, and tolerability of multiple doses of alogliptin benzoate (SYR-322), a dipeptidyl peptidase-IV inhibitor, in patients with type 2 diabetes [abstract]. Presented at: American Diabetes Association 67th Annual Scientific Sessions; June 22–26, 2007; Chicago, IL. Abstract 0499-P.
31. Defronzo RA, Hissa M, Blauwet MB, Chen RS. Saxagliptin added to metformin improves glycemia control in patients with type 2 diabetes [abstract]. Presented at: American Diabetes Association 67th Annual Scientific Sessions; June 22–26, 2007; Chicago, IL. Abstract 02850OR.
32. Januvia [package insert]. Whitehouse Station, NJ: Merck & Co; 2007.
33. Red Book. 2007 edition. Montvale, NJ: Thompson PDR; 2007.
34. Mentlein R. Dipeptidyl-peptidase IV (CD26)-role in the inactivation of regulatory peptides. Regul Pept. 1999;85:9–24.
