- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Dronedarone: An antiarrhythmic agent for the management of atrial fibrillation and atrial flutter
Dronedarone, an investigational antiarrhythmic agent being studied for the management of AF and atrial flutter, has a pharmacologic mechanism of action that is similar to that of amiodarone, but dronedarone lacks an iodine moiety, which may result in less thyroid and pulmonary toxicity. Dronedarone is currently pending FDA approval; the agent was granted priority review in August 2008.

Key Points
Abstract
Atrial fibrillation (AF) is a major cause of morbidity and mortality and affects approximately 2.5 million people in the United States. Patients suffering from AF have an increased risk of cardiovascular events and death and have a decreased heath-related quality-of-life. Dronedarone, an investigational antiarrhythmic agent being studied for the management of AF and atrial flutter, has a pharmacologic mechanism of action that is similar to that of amiodarone, but dronedarone lacks an iodine moiety, which may result in less thyroid and pulmonary toxicity. During clinical trials, dronedarone has been demonstrated to decrease AF recurrence by approximately 25% and to reduce the incidence of the combined end point of hospitalization for cardiac causes and all-cause mortality. However, the results of an additional trial suggest that dronedarone should not be used in patients with severe (class III or IV) heart failure, as these patients demonstrated an increased mortality risk with dronedarone treatment. Worsening renal function has also been associated with dronedarone, but the clinical relevance of this is a matter of some debate. Dronedarone is currently pending FDA approval; the agent was granted priority review in August 2008. (Formulary. 2009;44:40–46.)
CLINICAL PHARMACOLOGY
Dronedarone (also known as N-[2-butyl-3-[4-(3-dibutyl-aminopropoxy) benzoyl] methanesulfonamide, hydrochloride]) is a noniodinated benzofuran derivative of amiodarone with a sulfonamide group on the benzofuran ring (Figure 1).3,4 The electrophysiological properties of dronedarone are very similar to those of amiodarone; both agents belong to all 4 Vaughan-Williams classes. Consequently, amiodarone-like antiarrhythmic actions, including sodium-channel blocking at rapid pacing rates (class I effect), prolonged cardiac action potentials and refractoriness (class III effect), calcium-channel antagonism (class IV effect), and noncompetitive antiadrenergic effects (class II effect), have been demonstrated with dronedarone in dog, rat, and human hearts.4,5 As a result of these channel-blocking effects, there is a dose-related increase in the PR and QT intervals with dronedarone doses up to 1,600 mg/d.5–7 With the 400-mg twice daily dose typically used in clinical trials, the PR interval increased by 13.4 milliseconds, and the incidence of any QTc interval >500 milliseconds was 7.7%.5–7
PHARMACOKINETICS
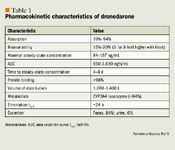
Dronedarone is metabolized via the cytochrome (CYP) P450 3A4 isoenzyme system, with its metabolites (1 metabolite identified as active but 3- to 10-fold less potent than dronedarone) excreted mainly in the feces. Only 6% of dronedarone is eliminated renally; consequently, drug levels are not expected to be influenced by changes in renal function.5 Clearance of dronedarone ranges from 130 to 150 L/h and is independent of the dose.5 Dronedarone and its active metabolite cross the blood-brain barrier and the placenta and are excreted into breast milk.5
CLINICAL TRIALS
Dronedarone has been studied in a number of randomized, double-blind, placebo-controlled trials enrolling patients with paroxysmal and persistent AF and those with differing classes of New York Heart Association (NYHA) heart failure (HF) and various degrees of cardiac risk (Table 2).6,8–15 To date, the results of 5 trials as part of 4 full-text publications are available for review.6,8,9,13 In addition, the results of a sixth trial were recently presented at the American Heart Association's and Heart Rhythm Society's 2008 Scientific Sessions.10–12,14,15 Each of these trials meets minimum standards for reporting of trial methodology and results (as depicted by each having a Jadad score ≥3).16
DAFNE. Dronedarone Atrial Fibrillation Study After Electrical Cardioversion (DAFNE) was the first published clinical trial of dronedarone and sought to determine optimal dosing.6 Patients (N=199) with persistent AF (duration, 72 h–12 mo) who were successfully cardioverted either electrically (73%–88% across groups) or pharmacologically were randomly allocated to treatment with dronedarone 400, 600, or 800 mg twice daily or placebo and were followed for 6 months. At the end of follow-up, recurrence of AF was lower among dronedarone-treated patients (35% still in NSR) than among placebo-treated patients (10% still in NSR), and the time to AF relapse was increased among patients treated with dronedarone 400 mg twice daily (median, 60 d) versus those treated with placebo (median, 5.3 d; P=.001). No significant additional effect was observed with higher dronedarone doses (specific data not reported). If AF did recur, patients who received dronedarone had lower ventricular rates compared with patients who received placebo (P=.0001). Spontaneous conversion to NSR in patients treated with dronedarone occurred in a dose-dependent fashion (5.8%, 8.2%, and 14.8% of patients treated with dronedarone 400, 600, and 800 mg twice daily compared with 3.1% of patients treated with placebo; P=.03). As a result of this study, the dronedarone 400 mg twice daily regimen was selected for further study.

In the combined EURIDIS/ADONIS analysis published by Singh et al,8 median times to recurrence of AF were reported to be 116 days in the dronedarone group and 53 days in the placebo group (P=not reported). At the final 12-month follow-up time point, the incidence of AF recurrence was lower in the dronedarone group than in the placebo group (HR=0.75; 95% CI, 0.65–0.87; P<.001). As in DAFNE, the ventricular rate in patients who developed AF was lower among dronedarone-treated patients than among placebo-treated patients (P<.001). Interestingly, patients receiving dronedarone were hospitalized or died 27% less often than patients who received placebo (P=.01). Although this was not the primary end point of the study, this result suggests that dronedarone may have great utility as an antiarrhythmic agent beyond maintaining NSR.
ANDROMEDA. The results of the Antiarrhythmic Trial with Dronedarone in Moderate-to-Severe Congestive Heart Failure Evaluating Morbidity Decrease (ANDROMEDA), a multicenter, randomized, double-blind, placebo-controlled trial of hospitalized patients with symptomatic HF and severe left ventricular systolic dysfunction, were published in 2008.9 In ANDROMEDA, patients hospitalized with new or worsening symptomatic HF (mainly NYHA classes II and III) received either dronedarone 400 mg twice daily or placebo to determine whether dronedarone could reduce the occurrence of the composite of death from any cause or hospitalization for HF. Although investigators had planned to include 1,000 patients in the initial study population and follow those patients for a minimum of 12 months, the safety monitoring board recommended the early termination of ANDROMEDA after only 627 patients (310 in the dronedarone group and 317 in the placebo group) were enrolled and followed for a median of 2 months.
A total of 25 patients (8.1%) in the dronedarone group and 12 patients (3.8%) in the placebo group died (HR=2.13; 95% CI, 1.07–4.25; P=.03). Worsening HF caused 10 deaths in the dronedarone group and 2 deaths in the placebo group, which suggests that dronedarone is not safe in patients with moderate-to-severe HF. Despite the increased mortality in the dronedarone group, the occurrence of the primary end point did not differ significantly between the 2 treatment groups (P=.12).
ATHENA. At Heart Rhythm 2008 (the Heart Rhythm Society's Scientific Sessions) and the 2008 American Heart Association Scientific Sessions, the results of Effects of Dronedarone on Cardiovascular Outcomes in High-Risk Patients with Atrial Fibrillation or Atrial Flutter (ATHENA) were presented.10–12,14,15 ATHENA was a placebo-controlled, double-blind, parallel-arm trial designed to assess the efficacy of dronedarone 400 mg twice daily (n=2,301) versus placebo (n=2,327) for the prevention of cardiovascular hospitalization or death from any cause in patients with AF. To be enrolled in ATHENA, patients were required to be at high risk for cardiac events and to have a history of paroxysmal or persistent AF or atrial flutter. High risk was defined by investigators as age >74 years or ≥1 of the following: hypertension, diabetes mellitus, prior stroke or transient ischemic attack or systemic embolism, enlarged left atrium, or a left ventricular ejection fraction <40%. Only 29% of patients had a history of HF, and patients with severe HF were excluded. Similar to ANDROMEDA, the primary study end point in ATHENA was the time to first cardiovascular hospitalization or death from any cause.
After a mean follow-up of 21 months, patients receiving dronedarone were less likely to experience the primary end point than patients receiving placebo (P<.001). The reduction in the primary end point for dronedarone versus placebo was mainly driven by reductions in cardiovascular hospitalization (HR reduction=25%; P<.001) and death caused by cardiac arrhythmic (HR reduction=45%; P=.01). Interestingly, it appeared that the reduction in cardiovascular hospitalizations with dronedarone was not caused only by reductions in AF hospitalizations (HR reduction=37%; P<.001) but also by reductions in non-AF-related hospitalizations (HR reduction=14%; P=.02).14 Furthermore, heart rate during AF decreased significantly with dronedarone compared with placebo (mean reduction in heart rate of 9 beats/min; P<.001), a result confirmed in the randomized, placebo-controlled Efficacy and Safety of Dronedarone for the Control of Ventricular Rate (ERATO) trial (mean reduction in heart rate of 11.7 beats/min; P<.001).13,15 These latter findings suggest that some of dronedarone's benefits may be a result of nonantiarrhythmic effects. These results build on those of EURIDIS and ADONIS and suggest that although dronedarone should not be used in patients with severe HF, patients with mild HF may still experience a mortality benefit.
ADVERSE EVENTS
The most common adverse effects associated with dronedarone are gastrointestinal in nature and include nausea or vomiting (4.3%) and diarrhea (7.1%).8 Adverse events and study withdrawals have been demonstrated to be dose-related.17
The most significant adverse events observed in clinical trials of dronedarone include a 113% increased hazard of death when the agent is administered to patients with severe, symptomatic HF.9 However, although administration of dronedarone appears to be problematic in patients with severe HF, patients with mild HF or who are at high cardiac event risk appear to have decreased mortality and/or need for cardiac hospitalization.8,10–12

As with other antiarrhythmic agents that block potassium channels (class III effect), dronedarone can cause QTc interval prolongation. At the 400-mg twice daily dose used in clinical trials, the incidence of any QTc interval >500 milliseconds was 7.7%.5–7 QTc interval prolongation has been associated with an increased risk of torsade de pointes (TdP). Dronedarone's predecessor and likely main market competition, amiodarone, has the lowest risk of TdP among class III antiarrhythmic agents because it induces less transmural dispersion of repolarization (a smaller differential of repolarization across the epicardial, endocardial, and midmyocardial layers) than other class Ia or III antiarrhythmic agents.20 Differences in dispersion of repolarization and risk of TdP with dronedarone have been evaluated in a dog model; dronedarone has been demonstrated to significantly increase transmural dispersion of repolarization compared with amiodarone and has been associated with several episodes of TdP.21 As such, the comparative risk of TdP with dronedarone versus amiodarone and other class Ia or III antiarrhythmics needs further evaluation, and caution is warranted in the interim.
In phase 1, 2, and 3 clinical trials, a 10% to 15% increase in serum creatinine levels has been observed in patients receiving dronedarone, raising concerns about the agent's potential nephrotoxicity.6,8–12,22 To address these concerns, Tschuppert et al22 conducted a study of dronedarone in healthy volunteers to assess the cause of the elevated creatinine levels in dronedarone recipients. They discovered that dronedarone affects the renal handling of creatinine (impaired cation transport) while leaving the glomerular filtration rate unchanged. Thus, these data suggest that dronedarone has a reversible effect on serum creatinine that should not be interpreted as an impairment of renal function. These data may also suggest the potential for drug interactions between dronedarone and other drugs undergoing tubular excretion such as amantadine, metformin, and procainamide.
DRUG INTERACTIONS
There is little published clinical information regarding drug interactions with dronedarone. Consequently, data for this review were obtained from the European Medicines Agency (EMEA) marketing authorization dossier for dronedarone.5
In vitro, dronedarone has been demonstrated to be metabolized (>84%) by the CYP3A4 isoenzymes and to be a moderate inhibitor of the CYP3A4 and CYP2D6 isoenzymes. In addition, the main active metabolite of dronedarone (SR 35021) has also been demonstrated to have the potential to inhibit CYP2C9, CYP2C19, and CYP1A2.
Effects of other drugs on dronedarone. The potent CYP3A4 inhibitor ketoconazole increases dronedarone exposure by 25-fold; therefore, co-administration of dronedarone with potent CYP3A4 inhibitors, such as ketoconazole, itraconazole, nefazodone, protease inhibitors (ritonavir), erythromycin, and clarithromycin, should be carried out with extreme caution or avoided. Healthcare professionals should consider initial lower dronedarone doses or downward dose adjustments of dronedarone when the agent is co-administered with moderate CYP3A4 inhibitors or with the heart-rate lowering (through negative chronotropic or dromotropic mechanisms) calcium antagonists (verapamil or diltiazem). Use of the potent CYP3A4 inducer rifampicin led to a 5-fold lower, clinically ineffective dronedarone exposure. As such, caution is warranted when dronedarone is used with potent enzyme inducers, as well.
Pantoprazole only increased dro- nedarone maximal concentration by 13%, suggesting that alterations in pH do not influence dronedarone bioavailability to a clinically important extent.
Effects of dronedarone on other drugs. Data suggest that concomitant administration of dronedarone and digoxin results in a 1.7- to 2.5-fold increase in maximal concentrations and the AUC of digoxin (likely mediated through a P-glycoprotein interaction), necessitating a 50% reduction in digoxin dose. Co-administration of dronedarone and simvastatin leads to 2- to 4-fold increases in simvastatin levels. This increased exposure to simvastatin may be clinically relevant and could lead to increased risk of statin dose-related adverse events such as myopathy and rhabdomyolysis. The administration of beta-blockers is influenced by dronedarone's inhibition of CYP2D6. Dronedarone increases metoprolol and propranolol exposure by approximately 1.6- and 1.3-fold, respectively. Although these effects are modest, because of the potential for both pharmacokinetic and pharmacodynamic (bradycardia and heart block) interactions, caution is warranted with concomitant use of dronedarone and beta-blockers. Although increased drug exposure has been observed with other CYP3A4 substrates such as verapamil (1.3-fold), nisoldipine (1.5-fold), and combined oral contraceptives (levonorgestrel, 1.2-fold; ethinylestradiol, 1.3-fold) when co-administrated with dronedarone, these interactions are not thought to be clinically relevant, although caution should be exercised with concomitant verapamil use as discussed earlier. Warfarin and losartan, CYP2C9 substrates, have demonstrated limited potential for interaction with dronedarone.
DOSING AND ADMINISTRATION
Based on available clinical trials, the dosage of dronedarone most likely to be used is 400 mg twice daily.6,8–12 Pharmacokinetic data suggest that no dosage adjustment is required based on age, sex, or kidney function.5 Data on the effect of hepatic impairment on dosing were not available at the time of writing. Since bioavailability appears to increase significantly with concomitant food ingestion, patients should be instructed to take dronedarone consistently under similar feeding conditions.5
Dr Coleman is assistant professor of Pharmacy Practice, University of Connecticut School of Pharmacy, and methods chief and program director, University of Connecticut/Hartford Hospital Evidence-Based Practice Center. Dr White is professor of Pharmacy Practice, University of Connecticut School of Pharmacy, and director, University of Connecticut/Hartford Hospital Evidence-Based Practice Center. Dr Baker is senior research scientist, University of Connecticut/Hartford Hospital Evidence-Based Practice Center.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, clinical manager and director of Drug Information, Department of Pharmacy Services, Hartford Hospital, Hartford, Conn, and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn; and by Craig I. Coleman, PharmD, assistant professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
EDITORS' NOTE: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
REFERENCES
1. Rosamond W, Flegal K, Furie K, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2008 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146.
2. McNamara RL, Tamariz LJ, Segal JB, Bass EB. Management of atrial fibrillation: Review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Intern Med. 2003;139:1018–1033.
3. Tafreshi MJ, Rowles J. A review of the investigational antiarrhythmic agent dronedarone. J Cardiovasc Pharmacol Ther. 2007;12:15–26.
4. Watanabe Y, Kimura J. Acute inhibitory effect of dronedarone, a noniodinated benzofuran analogue of amiodarone, on Na+/Ca2+ exchange current in guinea pig cardiac ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:371–376.
5. European Medicines Agency. Withdrawal public assessment report of the marketing authorisation application for Multaq (dronedarone). http://www.emea.europa.eu/humandocs/PDFs/EPAR/multaq/361489en4.pdf . Accessed January 19, 2009.
6. Touboul P, Brugada J, Capucci A, Crijns HJ, Edvardsson N, Hohnloser SH. Dronedarone for prevention of atrial fibrillation: A dose-ranging study. Eur Heart J. 2003;24:1481–1487.
7. Wadhani N, Sarma JS, Singh BN, Radzik D, Gaud C. Dose-dependent effects of oral dronedarone on the circadian variation of RR and QT intervals in healthy subjects: Implications for antiarrhythmic actions. J Cardiovasc Pharmacol Ther. 2006;11:184–190.
8. Singh BN, Connolly SJ, Crijns HJ, et al; EURIDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357:987–999.
9. Kober L, Torp-Pederson C, McMurray JJ, et al; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–2687.
10. Hohnloser SH. Effects of dronedarone on cardiovascular outcomes in high-risk patients with atrial fibrillation or atrial flutter-results of the ATHENA trial. Presented at: Heart Rhythm 2008; May 15, 2008; San Francisco, CA.
11. Coletta AP, Cleland JG, Cullington D, Clark AL. Clinical trials update from Heart Rhythm 2008 and Heart Failure 2008: ATHENA, URGENT, INH study, HEART and CK-1827452. Eur J Heart Fail. 2008;10:917–920.
12. Landmark ATHENA study findings with Multaq (dronedarone) show 24% reduction in cardiovascular hospitalisation or death in patients with atrial fibrillation [press release]. Paris, France: Sanofi-Aventis; May 15, 2008.
13. Davy JM, Herold M, Hoglund C, et al. Dronedarone for the control of ventricular rate in permanent atrial fibrillation: The Efficacy and Safety of Dronedarone for the Control of Ventricular Rate During Atrial Fibrillation (ERATO) study. Am Heart J. 2008;156:527.e1–527.e9.
14. Torp-Pedersen C, Page RL, Connolly SJ, et al. The effect of dronedarone on hospitalizations in patients with atrial fibrillation. Results from the ATHENA study. American Heart Association 2008 Scientific Sessions; November 8–12, 2008; New Orleans, LA. Abstract 4101.
15. Page RL, Connolly SJ; Crijns HJ, et al. Rhythm- and rate-controlling effects of dronedarone in patients with atrial fibrillation: Insights from the ATHENA trial. American Heart Association 2008 Scientific Sessions; November 8–12, 2008; New Orleans, LA. Abstract 4097.
16. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12.
17. Kowey PR, Singh BN. Dronedarone in patients with implantable defibrillators. Presented at: Heart Rhythm 2004; May 22, 2004; San Francisco, CA.
18. Quaglino D, Ha HR, Duner E, et al. Effects of metabolites and analogs of amiodarone on alveolar macrophages: Structure–activity relationship. Am J Physiol Lung Cell Mol Physiol. 2004;287:L438–L447.
19. Van Beeren HC, Jong WM, Kaptein E, Visser TJ, Bakker O, Wiersinga WM. Dronedarone acts as a selective inhibitor of 3,5,3'-triiodothyronine binding to thyroid hormone receptor-alpha1: In vitro and in vivo evidence. Endocrinology. 2003;144:552–558.
20. Van Opstal JM, Schoenmakers M, Verduyn SC, et al. Chronic amiodarone evokes no torsade de pointes arrhythmias despite QT lengthening in an animal model of acquired long-QT syndrome. Circulation. 2001;104:2722–2727.
21. Moro S, Ferreiro M, Celestino D, Medei E, Elizari MV, Sicouri S. In vitro effects of acute amiodarone and dronedarone on epicardial, endocardial, and M cells of the canine ventricle. J Cardiovasc Pharmacol Ther. 2007;12:314–321.
22. Tschuppert Y, Buclin T, Rothuizen LE, et al. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol. 2007;64:785–791.
FDA Approves Combination Therapy for Pulmonary Arterial Hypertension
March 25th 2024J&J’s Opsynvi is single-tablet combination of macitentan, an endothelin receptor antagonist, and tadalafil, a PDE5 inhibitor. It will be priced on parity with Opsumit, which is also a J&J product to treat patients with PAH.
FDA Issues Complete Response Letter for Onpattro in Heart Failure Indication
October 9th 2023Alnylam Pharmaceuticals will no longer pursue this indication of Onpattro and will instead on focus on a label expansion for Amvuttra, which is in phase 3 development to treat patients with cardiomyopathy of ATTR amyloidosis.
