- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
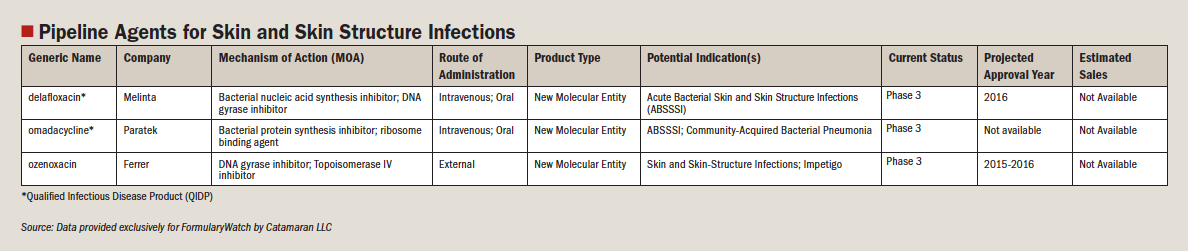
- Dermatology
- Oncology
Drugs in Perspective: Dalvance
Dalvance (dalbavancin) is the first and only intravenous antibiotic was approved on May 2014 to treat ABSSSIs with a 2-dose regimen of 1,000 mg once week and later on a 500 mg that is given over a 30-minute time span
Introduction
A skin and skin structure infection is recognized as a bacterial infection of the skin and the associated tissues and the infection can be viewed as being a complicated or uncomplicated skin and skin structure infection. 1 A complicated infection involves the deeper soft tissue and may require surgical intervention whereas the uncomplicated infection can be an abscess, cellulitis, or furuncles. Both terms have been referred to as acute bacterial skin and skin structure infections (ABSSSI).1 ABSSSI can include an array of diseases and severity of presentation. There is an increased resistance to both gram-positive and gram-negative bacteria with methicillin-resistant Staphylococcus aureus being one of the main culprits.1 If the infection is diagnosed early on it can be cured but if treatment is delayed or not provided there is the potential for serious complications to occur such as septicemia, nephritis, or carditis.2 There are a series of antibiotics that are used to treat or reduce the symptoms of bacterial skin and skin structure infections with new agents being approved.
Dalvance (dalbavancin) is the first and only intravenous antibiotic was approved on May 2014 to treat ABSSSIs with a 2-dose regimen of 1,000 mg once a week and later on a 500 mg that is given over a 30-minute time span.3 Dalbavancin is a semisynthetic lipoglyopeptide antibacterial that interferes with cell wall synthesis by binding to the D-alanyl-D-alanine terminus of the stem pentapeptide in nascent cell wall peptidoglycan, thus preventing cross-linking.4,5 It is an antibacterial in vitro against Staphylococcus aureus and Streptococcus pyogenes at concentrations similar to those sustained throughout treatment in humans treated according to the recommended dosage regimen.5 Dalbavancin is available as a 500-mg reconstituted solution for intravenous administration.6 For the treatment of ABSSSI in adults the recommendation is for a 2-dose regimen of dalbavancin that includes 1,000 mg followed 1 week later by 500 mg. The 2 doses are to be administered 30 minutes apart by intravenous infusion. In patients with renal impairment who have a creatinine clearance <30ml/min and not on hemodialysis the recommendation is for 750 mg followed 1 week later by 375 mg.6
Relevance
Dalbavancin is the first drug to receive the designation as a qualified infection disease product under the Generating Antibiotic Incentives Now (GAIN) Act of the FDA Safety and Innovation Act. Dalbavancin is recognized as an innovative treatment option for ABSSSI that provides an equally unique 2-dose regimen for this serious infection compared to the routine once-daily or twice-intravenous antibiotics.7 The inability to successfully treat ABSSSI has been a growing issue and the availability of dalbavancin offers the chance to achieve improved healthcare outcomes.
Studies
The FDA approval of dalbavancin was based on 2 randomized, double blind, double-dummy clinical trials (Trial 1 and Trial 2).8 The intent to treat population consisted of 1,312 randomized patients hospitalized with a positive diagnosis of ABSSSI. The patients were given 2 weeks of either a 2-dose regimen of intravenous dalbavancin (1,000 mg followed 1 week later by 500 mg) or intravenous vancomycin (100 mg or 15 mg/kg every 12 hours).There was the option to switch to oral linezolid after 3 days.8 The patients that received dalbavancin with a creatinine clearance of <30ml/min were given 750 mg followed by 37 5mg 1 week later.8 The primary end point of the 2 ABSSSI trials was clinical response rate in which the responders where identified as those patients that had no increase from baseline in lesion area 48 to 72 hours after the start of therapy and a temperature at or below 37.6°C with measurement. The responders were 83.3% for dalbavancin and 81.8% for vancomycin in Trial 1 while it was 76.8% for dalbavancin and 78.3% for vancomycin/linezolid in Trial 2. 8 The adverse side effects that were associated with dalbavancin were nausea, headache, and vomiting.
Potential advantages and disadvantages
The FDA approval of dalbavancin provides a new IV antibiotic for the treatment of ABSSSI.7,9 It can help to decrease or even eliminate the time that spent in the hospital and care being provided in outpatient settings. While dalbavancin is highly regarded as an antibiotic that has the potential to improve patient outcomes, it has been reported to cause serious hypersensitivity or anaphylactic reaction. Additionally, rapid intravenous infusion can cause reactions such as flushing, rash, or pruritus.7,9
Cost
The estimated cost of 1 vial of 500-mg solution (reconstituted) of dalbavancin is $1,788.10
Place in therapy
Dalbavancin is the first intravenous antibiotic to be approved by FDA for ABSSSI. It is a synthetic lipoglycopetitide, second-generation, semi-synthetic agent and similar to vancomycin which is a glycopeptide dalbavancin contains a glycopepetide backbone with a lipophilic side chain.10,11 Dalbavancin can be served for use in patients who present with serious skin and skin structure infection who have tried and failed other therapies.
Implications
The FDA approval of dalbavancin has implications for the improving the future treatment of ABSSSI. Dalbavancin is an antibiotic with bactericidal activity against gram-positive bacteria and thus has the potential to reduce the incidences of the most serious infections that can include Staphylococcus aureus, Streptococcus pyogenes, and MRSA (methicillin-resistant Staphylococcus aureus).11
Click to enlarge
References
Shah M & Shah H. Acute bacterial skin and skin structure infections:Current perspective. Indian J Dermatol. 2011; 56(5): 510–512.
Hedrick J. Acute bacterial skin infections in pediatric medicine:Current issues in presentation and treatment. Pediatr Drugs. 2003:5 Suppl.1;35-46.
PBR. Durata therapetics’s dalvance injection gets FDA approval. Retrieved from http://drugdelivery.pharmaceutical-business-review.com/news/durata-therapeutics-dalvance-injection-gets-fda-approval-260514-4276338 Accessed June 30, 2014.
Brooks M. FDA clears dalbavancin (dalvance) for acute skin infections. Medscape Medical News. Retrieved from http://www.medscape.com/viewarticle/825678 Accessed June 30, 2014.
MediLexicon.Dalvance (dalbavanin) Available at http://www.medilexicon.com/drugs/dalvance.php Retrieved July 1, 2014
Rx List. Dalvance. Retrieved from http://www.rxlist.com/dalvance-drug/indications-dosage.htm Accessed July 1, 2014.
GlobalNewsWire. FDA approves durata’s therapeutics dalvance for the treatment of acute bacterial skin and skin structure infections…. Retrieved from http://finance.yahoo.com/news/fda-approves-durata-therapeutics-dalvance-201753887.html;_ylt=AwrSbnjUZbNTGjYAy2NXNyoA;_ylu=X3oDMTE0dGk0dGw3BHNlYwNzcgRwb3MDMTMEY29sbwNncTEEdnRpZANWSVA0NzNfMQ-- Accessed July 1, 2014.
CenterWatch. Dalvance. Retrieved from http://www.centerwatch.com/drug-information/fda-approved-drugs/drug/1329/dalvance-dalbavancin Accessed July 1, 2014.
Root C. FDA approves durata’s dalvance for skin infections. Clinical Leader. Retrieved from http://www.clinicalleader.com/doc/fda-approves-durata-s-dalvance-for-skin-infections-0001 Accessed July 1, 2014.
Dalvance (Dalbavancin). Lexi-Comp Online, Lexicomp. Hudson, Ohio: Lexi-Comp, Inc.; May 28, 2014.
Matilda B. New drug dalvance wins fda approval to treat skin infections. Science World Report. Retrieved from http://www.scienceworldreport.com/articles/14940/20140524/new-drug-dalvance-wins-fda-approval-to-treat-skin-infections.htm. Accessed July 1, 2014.
FDA Issues Complete Response Letter for Pz-Cel to Treat Epidermolysis Bullosa
April 22nd 2024Prademagene zamikeracel is a cell therapy designed to incorporate the functional collagen-producing COL7A1 gene into a patient’s own skin cells. The FDA is asking for additional information on manufacturing practices.
