- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
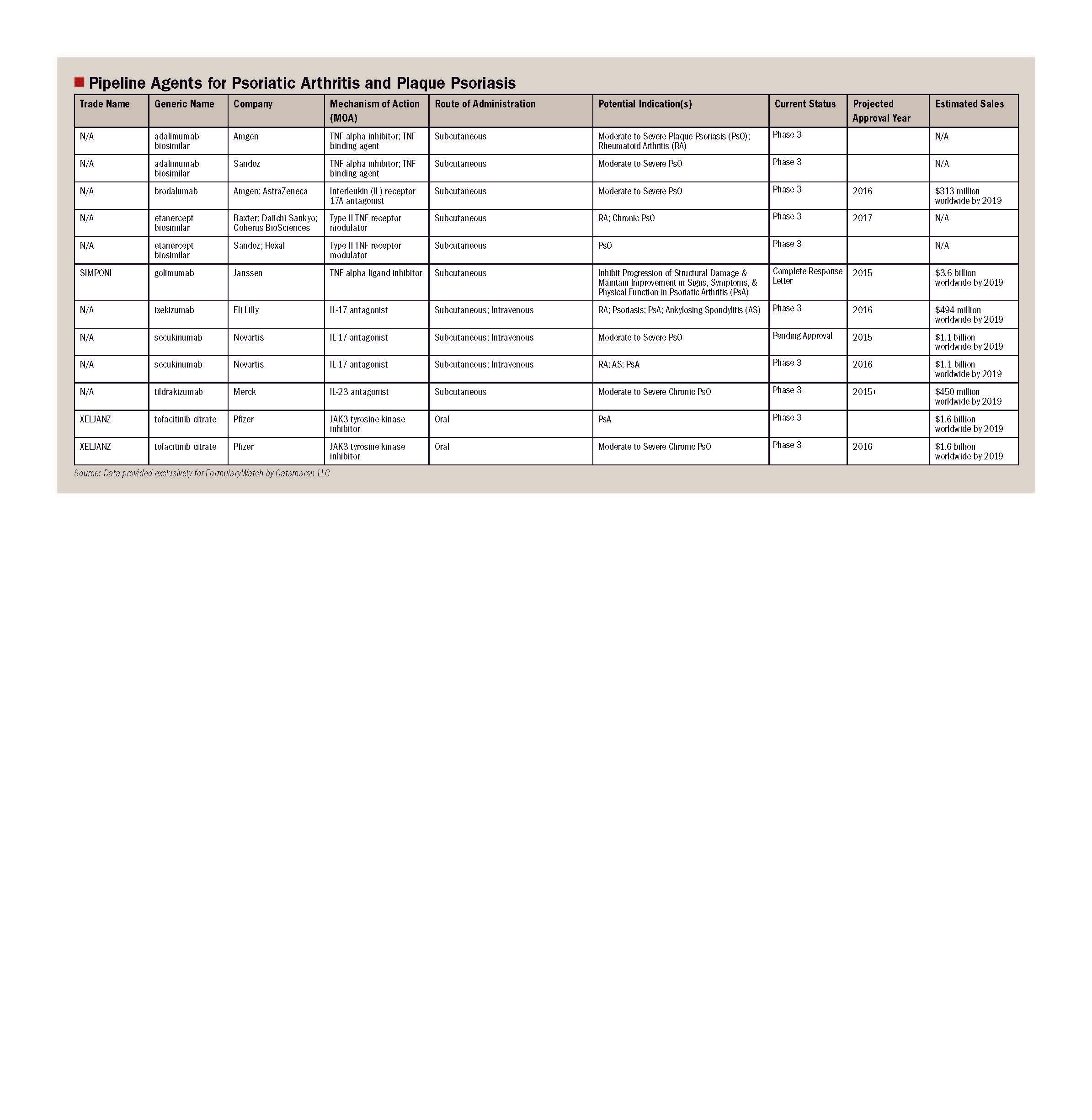
Drugs in Perspective: Otezla (apremilast)
Otezla (apremilast), a small-molecule inhibitor of phosphodiesterase 4 (PDE4), was approved by FDA on March 21, 2014, for the treatment of adult patients with active psoriatic arthritis. On September 23, 2014, it was approved for a second indication, the treatment of patients with moderate-to-severe plaque psoriasis who are candidates for phototherapy or systemic therapy
Psoriasis is a chronic, inflammatory disease that affects approximately 1% to 3% of the world population.1 Plaque psoriasis is the most common type of psoriasis, affecting approximately 80% to 90% of patients and is characterized by circular to oval, well-demarcated erythematous plaques. Psoriatic arthritis is a spondyloarthritic disease that occurs in approximately 30% of individuals with psoriasis and has an estimated prevalence of 0.1 to 1%. It is characterized by synovitis, enthesitis, dactylitis, and spondylitis. Although there is likely a genetic component, the exact etiology of psoriasis and psoriatic arthritis is unknown. Both share common physiologic mechanisms. Psoriasis, however, typically precedes psoriatic arthritis by approximately 5 to 10 years in most patients.2 Patients with psoriasis and psoriatic arthritis generally experience decreased productivity, functionality, and quality of life, potentially resulting in work absenteeism and long-term disability.1,3
Otezla (apremilast), a small-molecule inhibitor of phosphodiesterase 4 (PDE4), was approved by FDA on March 21, 2014, for the treatment of adult patients with active psoriatic arthritis.4,5 On September 23, 2014, it was approved for a second indication, the treatment of patients with moderate-to-severe plaque psoriasis who are candidates for phototherapy or systemic therapy.5,6 Apremilast inhibits PDE4, resulting in increased intracellular cyclic adenosine monophosphate (cAMP) levels. The specific mechanism by which apremilast exerts its therapeutic action in patients with psoriasis and psoriatic arthritis is not well defined.5
Apremilast is available as 30-mg oral tablets and a 2-week starter blister pack containing 10 mg, 20 mg, and 30 mg. Apremilast should be titrated to a maintenance dose of 30 mg orally twice daily with or without food (See Table 1 for titration schedule). Dose should be reduced to 30 mg once daily in patients with severe renal impairment (creatinine clearance <30 mL/min using Cockcroft-Gault equation), and only the morning dose titration schedule should be used in these patients (evening doses should be excluded). There are no dose adjustments for patients with hepatic impairment.5
Table 1 Apremilast titration schedule
AM dose
PM dose
10 mg
-
10 mg
10 mg
10 mg
20 mg
20 mg
20 mg
20 mg
30 mg
30 mg
30 mg
Source: Ref 5
Relevance
Apremilast, a first-in-class oral PDE4 inhibitor, represents a new oral treatment option for adult patients with moderate-to-severe plaque psoriasis and psoriatic arthritis. Current treatment options for plaque psoriasis include topical agents, phototherapy, and systemic agents. Current systemic treatment options for psoriatic arthritis include nonsteroidal antiinflammatory drugs (NSAIDs), disease-modifying antirheumatic drugs (DMARDs), and biologics, such as Stelara (ustekinumab) and tumor necrosis factor-alpha (TNF-α) inhibitors.1,7
Studies
FDA approval for psoriatic arthritis was based on results from 3 multicenter, randomized clinical trials: the PALACE 1, 2, and 3 trials.8 In the trials, 1,493 patients with persistent symptoms despite treatment with DMARDs, biologics, or both were randomized 1:1:1 to placebo, apremilast 20 mg twice daily, or apremilast 30 mg twice daily (with a titration period). Continued use of stable doses of methotrexate, leflunomide, sulfasalazine, low-dose oral corticosteroids, and NSAIDs was permitted, but topical therapy was not permitted. Notably, failure of biologics was limited to 10% or fewer of patients in PALACE 1 (details not available for PALACE 2 or 3). Primary end point in all 3 trials was the percentage of patients achieving a 20% or greater improvement in symptoms, as defined by American College of Rheumatology (ACR) response criteria (ACR20). Fifty percent and 70% improvement (ACR50 and ACR70, respectively) were also measured. Results of these trials are presented in Table 2.5,9 The most commonly reported adverse events in the PALACE trials were diarrhea, nausea, headache, and upper respiratory tract infection. Gastrointestinal adverse effects presented early and were self-limited. Available data up to 52 weeks from the PALACE trials suggest durability of the ACR20 response.9-11
Table 2 Results of PALACE 1, 2, and 3 trials
PALACE 1
PALACE 2
PALACE 3
Placebo
(%)
n=168
Apremilast
20 mg (%)
n=168
Apremilast
30 mg (%)
n=168
Placebo
(%)
n=159
Apremilast
30 mg (%)
n=162
Placebo
(%)
n=169
Apremilast
30 mg (%)
n=167
19
30*
38*
19
32*
18
41*
6
-
16
5
11
8
15
1
-
4
1
1
2
4
*Statistically significant vs placebo
Source: Refs 5,9
FDA approval for moderate-to-severe plaque psoriasis was based on results from the ESTEEM trials.6 In the trials, 1257 patients with moderate-to-severe plaque psoriasis were randomized 2:1 to apremilast 30 mg twice daily (with a titration period) or placebo. The primary end point was the number of patients with a 75% improvement on the Psoriasis Area and Severity Index (PASI-75). In ESTEEM 1, significantly more patients receiving apremilast achieved PASI-75 compared to placebo (33.1% vs 5.3%; P<.0001) at 16 weeks. In ESTEEM 2, significantly more patients receiving apremilast also achieved PASI-75 compared to placebo (28.8% vs 5.8%; P<.0001) at 16 weeks.12,13 Notably, key trials with Enbrel (etanercept) have found that approximately 38% to 40% of patients achieve PASI-75 within a similar timeframe. Key trials with Remicade (infliximab) and Humira (adalimumab) have found that approximately 60% to 64% and 49% to 59%, respectively, achieve PASI-75 within a similar time frame.14 In the ESTEEM trials, the majority of adverse events with apremilast were considered mild to moderate in severity and consisted primarily of nausea and vomiting, which generally resolved within 1 month.12,13
Potential advantages and disadvantages
Limitations to current treatment options include potential adverse effects related to individual medications (eg, liver toxicity or interstitial lung disease with methotrexate), lack of long-term data with DMARDs to suggest delay or improvement in radiographic damage, and boxed warnings associated with individual medications (eg, increased risk of serious infections). Cost and route of administration may also be limiting factors for some patients. Although not directly compared, findings comparing biologics to placebo with ACR20 and PASI-75 endpoints suggest that biologics may have greater efficacy than apremilast, and despite being a small molecule agent, apremilast is priced in a similar range as biologically derived therapies.7,14,15 In addition, apremilast carries a warning for depression because cases of depression, including instances of observed suicidal ideation and behavior, were reported in clinical trials. Other warnings and precautions associated with apremilast include decreased weight and drug interactions.5
Cost
The wholesale acquisition cost (WAC) and average wholesale price (AWP) are $1875.00 and $2250.00, respectively, for 60 tablets of 30 mg apremilast. The WAC and AWP for the apremilast 2-week starter packs are $843.75 and $1012.50, respectively.15
Place in therapy
The goal of treatment for patients with psoriasis is to induce and maintain remission. According to the American Academy of Dermatology, topical preparations alone or in combination with phototherapy are recommended before using biologic or systemic therapy for the treatment of psoriasis, although stepwise therapy is not required.3,16-20 Topical therapies are the mainstay for mild plaque psoriasis either as monotherapy or in combination, and are also commonly used in conjunction with phototherapy, traditional systemic agents, or biologic agents for moderate-to-severe disease.17 Oral antipsoriatic systemic agents are typically reserved for moderate-to-severe psoriasis and are often combined with other therapies.18
NSAIDs and DMARDs are often used prior to biologic agents in the treatment of psoriatic arthritis. Mild forms of psoriatic arthritis typically respond to NSAIDS. Moderate disease tends to be unresponsive to NSAIDS and typically requires treatment with DMARDS or biologics. Severe psoriatic arthritis may require both DMARDs and biologic therapies.16,18,21,22
Apremilast has not yet been addressed in published guidelines. This agent has demonstrated safety and efficacy in the treatment of psoriatic arthritis and plaque psoriasis. At the same time, however, apremilast has not been compared to other approved treatments in published clinical trials, and results from placebo-controlled trials suggest a degree of efficacy that may be less than most FDA-approved biologic alternatives. It has been used with and without DMARDs, specifically methotrexate, leflunomide, and sulfasalazine, for the treatment of psoriatic arthritis in clinical trials.9-11 Apremilast likely offers an oral alternative to biologics in patients not responding adequately to biologics, those with a diminished response over time, and those who are unable to take or tolerate the biologic agents.
Implications
Through its unique mechanism of action and oral availability, apremilast offers adult patients with plaque psoriasis and psoriatic arthritis an additional therapeutic option for the management of these conditions. With a price comparable to commonly utilized biologic agents, further clinical trial data and real-world experience are required to assess this agent’s true value versus other agents in the management of its FDA-approved indications.
Dr Frick is a clinical pharmacist with the Drug Intelligence Department at Catamaran.
Disclosure information: The author reports no financial disclosures as related to products discussed in this article.
References
1. Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74(4):423–441.
2. American Academy of Dermatology. Position statement for maintenance therapy for psoriasis patients. August 18, 2012. http://www.aad.org/Forms/Policies/Uploads/PS/PS-Maintenance%20Therapy%20for%20Psoriasis%20Patients.pdf. Accessed October 20, 2014.
3. Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 6: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65(1):137–174.
4. FDA news release. FDA approves OTEZLA to treat psoriatic arthritis. March 21, 2014. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm390091.htm. Accessed October 20, 2014.
5. OTEZLA [prescribing information]. Summit, NJ: Celgene Corporation; September 2014.
6. Celgene Corporation press release. Oral Otezla (apremilast) approved by the U.S. Food and Drug Administration for the treatment of patients with moderate to severe plaque psoriasis. September 23, 2014. http://ir.celgene.com/releasedetail.cfm?ReleaseID=872240. Accessed October 20, 2014.
7. Belge K, Brück J, Ghoreschi K. Advances in treating psoriasis. F1000Prime Rep. 2014;6:4.
8. Celgene Corporation press release. Otezla (apremilast) – first oral therapy approved by the U.S. Food and Drug Administration for the treatment of adults with active psoriatic arthritis. March 21, 2014. http://ir.celgene.com/releasedetail.cfm?ReleaseID=834687. Accessed October 20, 2014.
9. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73(6):1020–1026.
10. Cutolo M, Myerson GE, Fleischmann RM, et al. Long-term (52-week) results of a phase 3, randomized, controlled trial of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis (PALACE 2). Abstract session: The American College of Rheumatology and Association of Rheumatology Health Professionals Annual Meeting; 2013 October 25–30; San Diego, CA.
11. Edwards CJ, Blanco FJ, Crowley J, et al. Long-term (52-week) results of a phase 3, randomized, controlled trial of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement (PALACE 3). Poster session: The American College of Rheumatology and Association of Rheumatology Health Professionals Annual Meeting; 2013 October 25–30; San Diego, CA.
12. Papp K, Griffiths C, Leonardi C, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe psoriasis: results from the randomized treatment withdrawal phase of a phase 3, randomized, controlled trial (ESTEEM 1). Poster session: The American Academy of Dermatology 72nd Annual Meeting; 2014 March 21–25; Denver, CO.
13. Paul C, Crowley J, Cather J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe psoriasis: 16-week results of a phase 3 randomized, controlled trial (ESTEEM 2). Poster session: The American Academy of Dermatology 72nd Annual Meeting; 2014 March 21–25; Denver, CO.
14. Huynh D, Kavanaugh A. Psoriatic arthritis: current therapy and future approaches. Rheumatology (Oxford). 2014; Aug 14. Epub ahead of print.
15. PriceRx [internet database]. Medispan. Updated periodically. https://pricerx.medispan.com/. Accessed September 14, 2014.
16. Gottlieb A, Korman NJ, Gordon KB, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 2: psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol. 2008;58(5):851–864 (update in progress).
17. Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3: guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60(4):643–659.
18. Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 4: guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61(3):451–485.
19. Menter A, Korman NJ, Elmets CA, et al; American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 5: guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62(1):114–135.
20. American Academy of Dermatology. Position statement on treatment of psoriatic patients. April 27, 2013. http://www.aad.org/Forms/Policies/Uploads/PS/PS%20on%20Treatment%20of%20Psoriatic%20Patients.pdf. Accessed October 20, 2014.
21. Ritchlin CT, Kavanaugh A, Gladman DD, et al; Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA). Treatment recommendations for psoriatic arthritis. Ann Rheum Dis. 2009;68(9):1387–1394.
22. Gossec L, Smolen JS, Gaujoux-Viala C, et al. European League Against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies. Ann Rheum Dis. 2012;71(1):4–12.
