- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Drugs in Perspective: Sovaldi (sofosbuvir)
Hepatitis C is a viral disease that causes the inflammation of the liver which can ultimately lead to a diminished liver function or liver failure. The majority of individuals who are infected with HCV do not present with any symptoms of the disease until the appearance of liver damage. According to the Centers for Disease Control and Prevention it is estimated that approximately 3.2 million American are infected with hepatitis C and globally about 150 million people have hepatitis C. Up to 85% of those who are initially infected with HCV will not eliminate the virus and become chronically infected.
Hepatitis C is a viral disease that causes the inflammation of the liver which can ultimately lead to a diminished liver function or liver failure.1 The majority of individuals who are infected with HCV do not present with any symptoms of the disease until the appearance of liver damage. According to the Centers for Disease Control and Prevention it is estimated that approximately 3.2 million Americans are infected with hepatitis C and globally about 150 million people have hepatitis C.1 Up to 85% of those who are initially infected with HCV will not eliminate the virus and become chronically infected.2
Sovaldi (sofosbuvir), an oral tablet is a direct acting antiviral agent and the first-in-kind nucleotide analog inhibitor that was approved by FDA on December 6, 2013 for the treatment of adults with chronic hepatitis C infection.3 Sovaldi works to inhibit HCV NS5B RNA dependent RNA polymerase which is considered to be an essential component of viral replication. It undergoes a metabolism to form the active uridine analog triphosphate (GS-461203) that is combined into HCV RNA by NS5B polymerase.4
It is available as a 400-mg once-daily tablet with or without food.4,5 It can be taken in combination with ribavirin or in combination with pegylated interferon and ribavirin for the treatment of chronic hepatitis C.5 The treatment regimen and duration of treatment is generally based on the HCV genotype (genotype 1-4) and or clinical scenarios. There is no dosage adjustment needed for those with a CrCl >30 ml/min, no studies for those with CrCl <30, or those with decompensated cirrhosis. 5,6
Relevance
Sovaldi is recognized as a nucleotide analog inhibitor that blocks a particular protein that is needed by hepatitis C for replication. It is one of two drugs approved by FDA within a 2-week time span to treat chronic HCV infection, with Olysio (simeprevir) being approved on November 22, 2013. 7 Like Sovaldi, Olysio can be used in combination with peginterferon alfa and ribavirin, however Sovaldi is also the first approved interferon-free treatment regimen for people with HCV genotypes 2 and 3. The approval of Sovaldi offers the opportunity for improved treatment outcomes for individuals diagnosed with hepatitis C who may not have received prior treatment or have been non-responders to other treatments.7
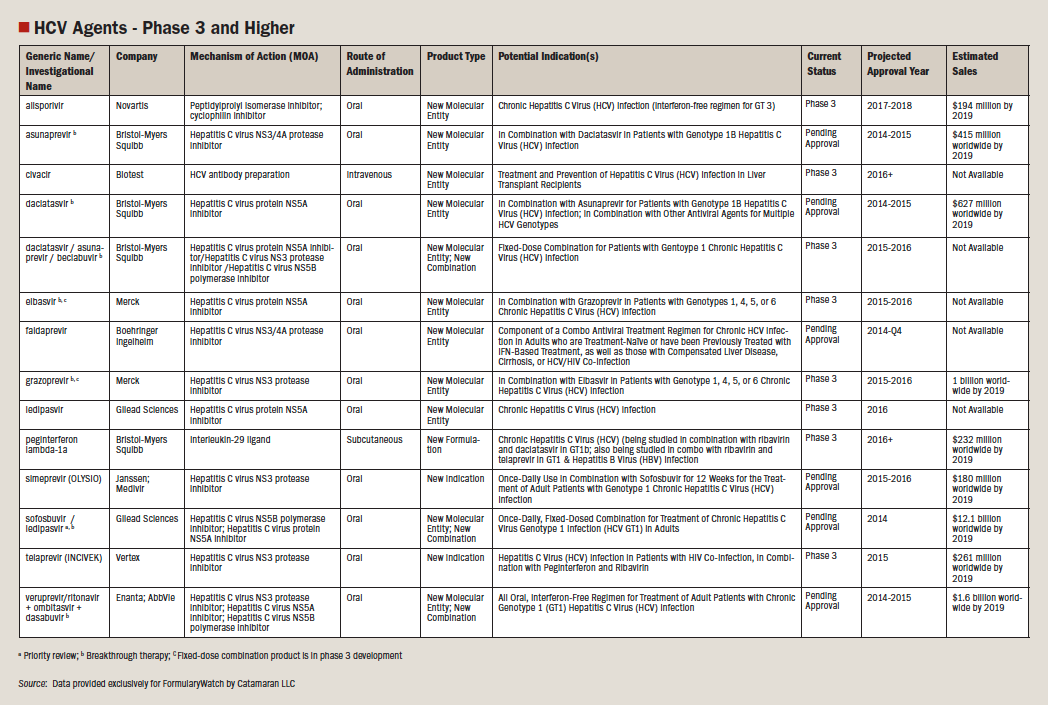
Studies
The effectiveness and approval of Sovaldi was based on 6 clinical trials with the end point of all trials being sustained virologic response (SVR), which was defined as HCV RNA less than LLOQ (lowest HCV RNA concentration that is within the validated quantitative range of an assay) at 12 weeks after the end of treatment. 8 The trials consisted of 1947 participants, with a subset being on trials of Sovaldi plus ribavirin with HCV genotype 2 or 3 (including both treatment naïve patients or those who failed or were unable to tolerate previous therapy) and others given triple combination of Sovaldi, ribavirin, and peginterferon alfa in 3 treatment naïve patients that were infected with HCV genotypes 1, 4, 5, or 6.9
Potential advantages and disadvantages
The approval of Sovaldi sets the stage for an exciting time in the treatment of hepatitis C infection as it is the first all oral interferon-free regimen. There is a movement away from the use of interferon and the possibility of a cure for most individuals with hepatitis C being a reality. While novel in its approach to treating hepatitis C, which is without the need for co-administration with interferon. Sovaldi can be associated with adverse effects when used in combination with ribavirin such as fatigue and headache and anemia and insomnia when used in combination with peginterferon alfa.8,10
Cost
The cost of a 28 count of 400 mg of Sovaldi is estimated at about $84,000 for 12 weeks of treatment.11 The pricing of Sovaldi, at more than $1,000 per tablet, has created significant angst amongst both activists and payer. When examining current costs of treatment based upon cost per SVR, Sovaldi may however represent cost-effectiveness advantages versus current standards of care when administered over 12 weeks in genotype 1 and 2 patients. 12 The cost per SVR in genotype 3 patients treated with sofosbuvir plus ribavirin ($312,357) is more than double than peginterferon plus ribavirin ($128,508).12
Place in Therapy
The approval and subsequent availability of Sovaldi provides a welcomed addition to the current armamentarium of options for the treatment of patients with hepatitis C infection. Sovaldi delivers an enhanced SVR versus existing treatment options in patients with various genotypes, and is the first FDA-approved all oral, interferon-free regimen for patients diagnosed with genotype 2 and 3 chronic hepatitis C.
Implications
FDA approval of Sovaldi has significant implications for the future direction of hepatitis C treatment. Sovaldi and other newer drugs work to inhibit the enzymes that are produced by the hepatitis C virus and allow for the potential for improved treatment outcomes. The observed cure rates identified with Sovaldi are over 80% with the success being dependent on the strain or genotype of the involved virus. The approval of Sovaldi represents a tremendous shift in the treatment direction for individuals with chronic hepatitis C.
Dr Farinde serves on the faculty at Columbia Southern University, Orange Beach, Ala.
Disclosure information: The author reports no financial disclosures as related to products discussed in this article.
References
FDA. FDA approves solvadi for chronic hepatitis C. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm377888.htm Accessed March 20, 2014.
Nettleman M, Rajaguru S, & Marks J. Hepatitis C Infection (HCV, Hep C).Available at http://www.medicinenet.com/hepatitis_c/article.htm Accessed March 20, 2014.
Tucker M. FDA approve game changer hepatitis C drug sofosbuvir. Medscape Medical News. 2013. Available at http://www.medscape.com/viewarticle/817371
MPR.Solvadi rx. New Drug Product. Available at http://www.empr.com/sovaldi/drugproduct/304/. Accessed March 20, 2014.
Solvadi. Highlights of prescribing information. Available at http://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/sovaldi/sovaldi_pi.pdf Accessed March 20, 2014.
Rx List. Sovaldi. 20143 Available at http://www.rxlist.com/sovaldi-drug.htm Accessed March 20, 2014.
FDA. Approval of sovali (sofosbuvir) tablets for the treatment of chronic hepatitis C. Available at http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/ucm377920.htm
Centerwatch. Sovaldi.Available at http://www.centerwatch.com/drug-information/fda-approved-drugs/drug/1298/sovaldi-sofosbuvir Accessed March 20, 2014.
Gilead Sciences.U.S Food and drug administration approves Gilead’s sovaldi (sofobuvir) for the treatment of chronic hepatitis C. Available at http://www.businesswire.com/news/home/20131206005775/en/U.S.-Food-Drug-Administration-Approves-Gilead%E2%80%99s-Sovaldi%E2%84%A2#.UyuRIk7n_mI Accessed March 20, 2014.
PBR. Gilead Sciences receives FDA approval for chronic hepatitis C drug. Available at http://regulatoryaffairs.pharmaceutical-business-review.com/news/gilead-sciences-receives-fda-approval-for-chronic-hepatitis-c-drug-091213-4142974 Accessed March 20, 2014
Sovaldi (Sofobuvir). Lexi-Comp Online, Lexicomp. Hudson, Ohio: Lexi-Comp, Inc.; March 20, 2014.
Catamaran. Economic evaluation of the treatment of hepatitis C infection genotype 1. 2014.
McCroy W. FDA approves new hepatitis C drug. Edge. Available at http://www.edgeonthenet.com/health_fitness/health/Features/153254/fda_approves_new_hepatitis_c_drug
FDA Issues Complete Response Letter for Pz-Cel to Treat Epidermolysis Bullosa
April 22nd 2024Prademagene zamikeracel is a cell therapy designed to incorporate the functional collagen-producing COL7A1 gene into a patient’s own skin cells. The FDA is asking for additional information on manufacturing practices.
