- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Drugs to Watch: Gene Therapies
Cell and gene therapies can be used for patients with cancer and serious diseases who have limited or no treatment options — but at a cost.
Innovation in gene and cell therapies is not slowing down. The IQVIA Institute for Human Data Science expects that 55 to 65 cell-, gene- and RNA-based therapies will launch globally by 2027, and U.S. spending on such therapies could rise to $12 billion by then.
Curative, one-time cell and gene therapies are providing options for patients with cancer and rare disease. In June 2023, two gene therapies were approved for treating patients with Duchenne muscular dystrophy (Elevidys) and hemophilia A (Roctavian). Gene therapies, however, have high price tags. Roctavian, for example, has a price of $2.9 million and Elevidys is $3.2 million.
Elevidys (delandistrogene moxeparvovec-rokl), which was granted an accelerated approval, is a therapy that delivers a gene to muscle that codes for a shortened, functional form of dystrophin, which is mutated in DMD. In August, Sarepta Therapeutics, the therapy’s developer, announced that the first patient received Elevidys.
A cost-effectiveness analysis sponsored by Sarepta indicated the value of Elevidys between $5 million and $13 million compared with standard of care alone, with a willingness-to-pay threshold of $500,000. The analysis found that Elevidys shows increases to quality of life and to equal value of life years gained, a measure used to determine how much a treatment can extend life.
This is the transport vehicle of Roctavian, created from a naturally occurring virus. It delivers the functional gene via a one-time infusion into the body. Once inside the body, the transport vehicle releases the working F8 gene into liver cells and is designed to instruct those cells to produce factor VIII.
Photo courtesy of BioMarin
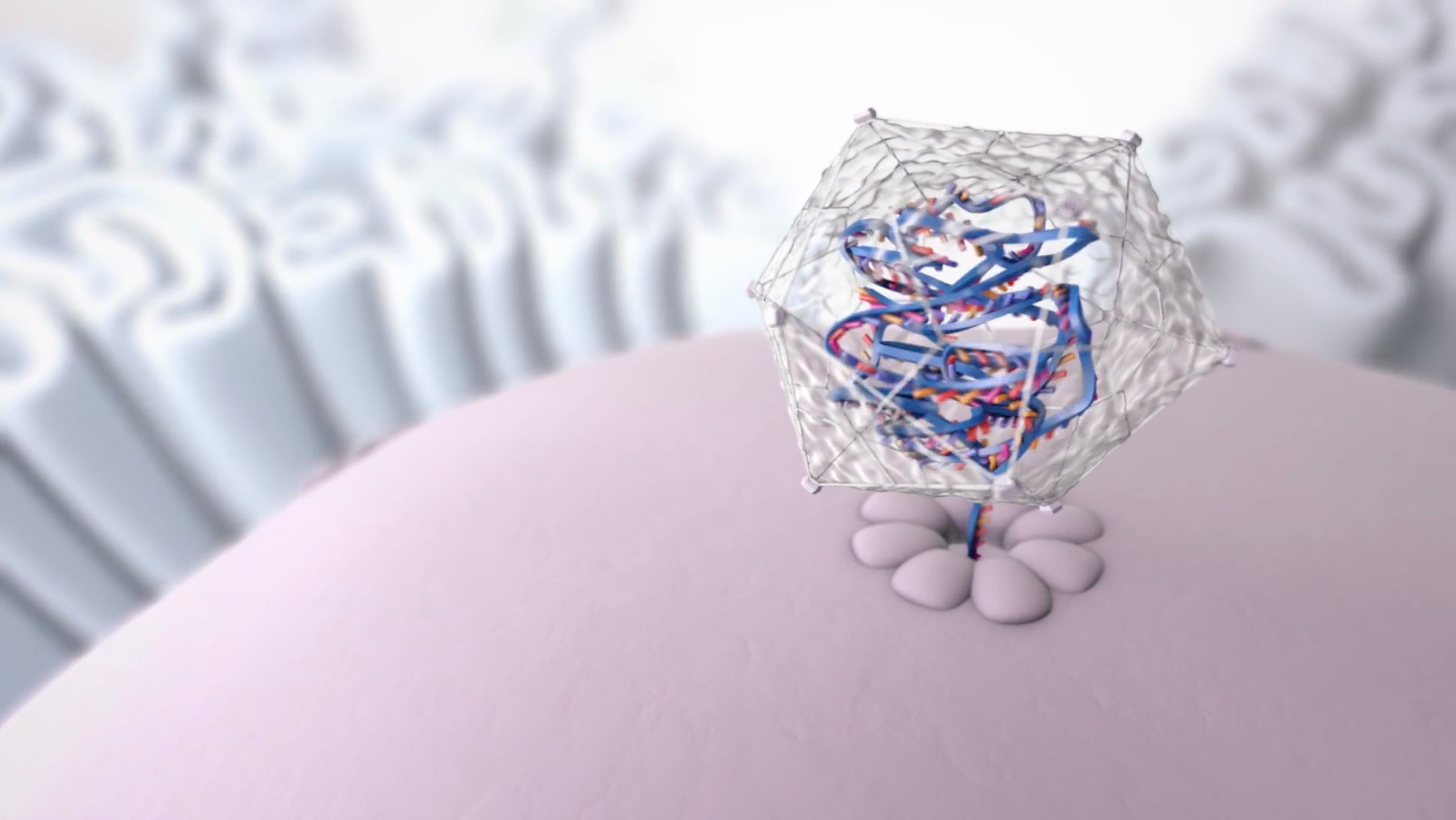
BioMarin’s Roctavian (valoctocogene roxaparvovec-rvox) was approved to treat adults with severe hemophilia A. It is designed to replace the function of the mutated gene, allowing people with severe hemophilia A to produce their own factor VIII. Company officials indicated that Roctavian adds value with both the clinical benefit and the economic benefit it offers. Treatments costs for patients with hemophilia A are about $800,000 per year for a typical patient.
BioMarin is offering an outcomes-based warranty that will reimburse payers up to 100% of the wholesale acquisition cost if a person does not respond to Roctavian. If the patient treated with Roctavian loses response at any time in the first four years, BioMarin will reimburse payers on a prorated basis for the cost of treatment.
The Institute for Clinical and Economic Review (ICER) released a revised evidence report in December 2022 that found Roctavian led to cost savings and projected gains in quality-adjusted life years. ICER analysts, however, said there are concerns about whether the benefit was short-lived and about the impact of the therapy on liver function and the risk for hepatocellular carcinoma. In a recent investor call, however, BioMarin officials said that in a phase 2 trial Roctavian remained durable for seven years
In the remainder of this year, we could see at least three more gene-based therapies, including two for sickle cell disease and another for advanced melanoma. One of these therapies is exagamglogene autotemcel (exa-cel) to treat patients with sickle cell disease; if approved, would be the first CRISPR-based, gene-edited therapy.
Sickle cell disease results from mutations in a gene that encodes a key component of hemoglobin, the oxygen-carrying molecule in blood. Current treatments for sickle cell include blood transfusions and medication that helps prevent sickling of red blood cells, reduce complications and manage pain. But a blood and bone marrow transplant is the only cure. This involves using donor cells, which could mean rejection.
CRISPR is a family of DNA sequences that, when coupled with the enzyme Cas9, acts as scissors to cut strands of DNA. The cell can recognize damaged DNA and works to repair it. This process enables scientists to edit parts of the genome. Shown here is CRISPR-Cas9 interacting with DNA.
Photo courtesy of Vertex Pharmaceuticals
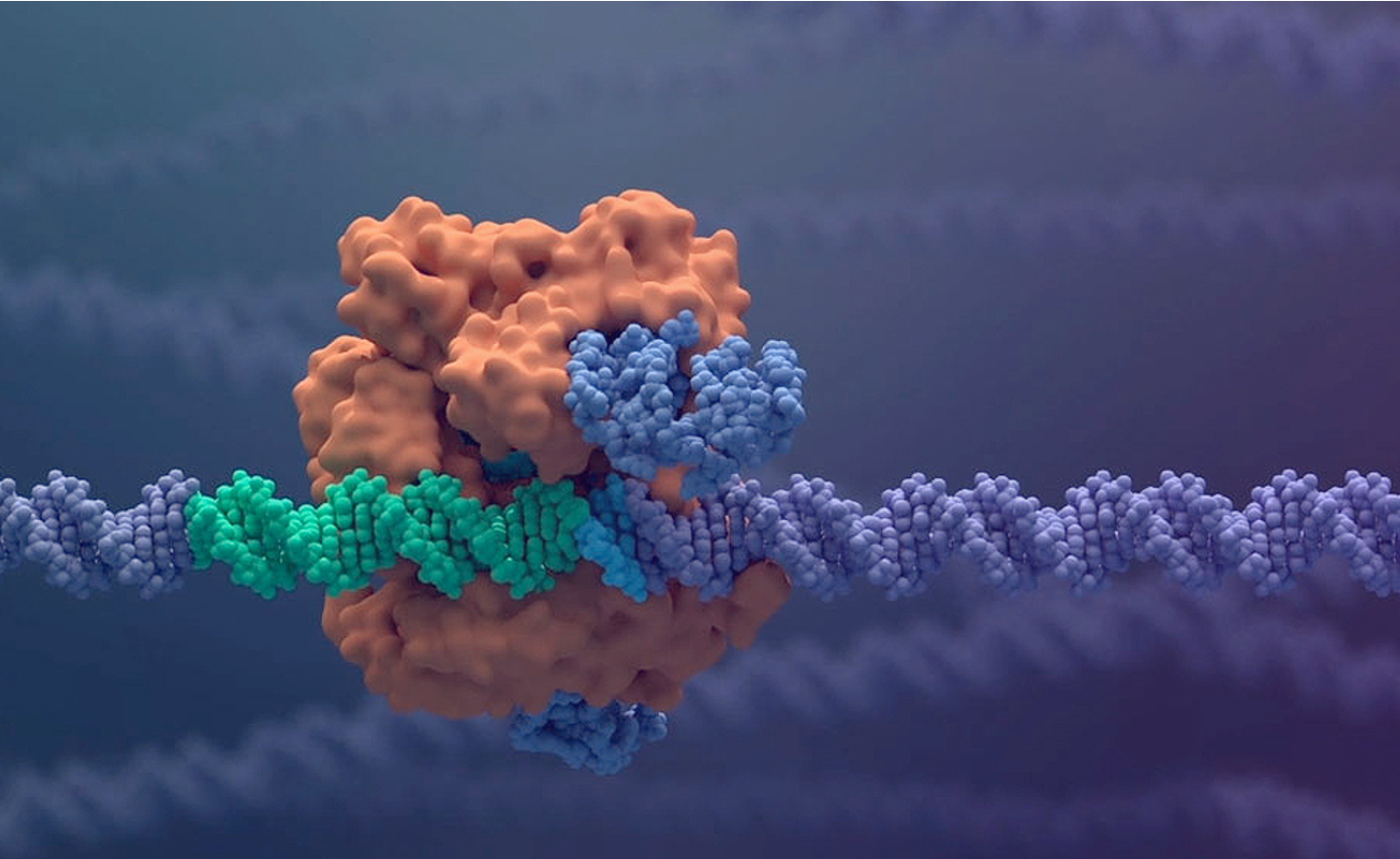
Developed by Vertex Pharmaceuticals Inc. and CRISPR Therapeutics, exa-cel is a one-time therapy that uses a patient’s hematopoietic stem cells, which are edited to produce high levels of fetal hemoglobin in red blood cells. The edited cells are infused into the patient as part of a stem cell transplant. The FDA has set the target action dates of Dec. 8, 2023, for exa-cel for use in patients with severe sickle cell disease and March 30, 2024, for patients with transfusion-dependent beta-thalassemia, a blood disorder that reduces production of red blood cells.
In updated results from two trials with 83 patients (CLIMB-111 and CLIMB-121), exa-cel met primary and key secondary end points. In patients with sickle cell, 94.1% achieved the primary end point of freedom from vaso-occlusive crises (VOC) for at least 12 consecutive months. VOC, or acute pain, occurs when sickled red blood cells block blood flow and tissues become deprived of oxygen. Vertex has started discussions with about 50 treatment centers in the United States that would be authorized to administered exa-cel.
Another potential new gene therapy for sickle cell disease is lovotibeglogene autotemcel (lovo-cel) from Bluebird Bio. The FDA has set an action date of Dec. 20, 2023. Lovo-cel is a one-time treatment designed to add functional copies of a modified form of the beta-globin gene into a patient’s blood stem cells, according to a news release from Bluebird Bio. Once patients have the beta-A-T87Q-globin gene, their red blood cells can produce anti-sickling hemoglobin, according to the release.
The regulatory submission is based on efficacy results from 38 patients and safety data from 50 patients. In one study of 32 patients, 96% of patients experienced complete resolution of severe vaso-occlusive events through 24 months.
The gene therapies for sickle cell disease would be cost-effective if priced between $1.35 million and $2.05 million, according to an evidence report by ICER released in July. ICER analysts found that both therapies are likely to improve quality and length of life, using a $2 million placeholder price for both.
Reviewers said, however, that the improvement seen with lovo-cel and exa-cel needs to be balanced against potential harms of myeloablative conditioning, which is the use of high-dose chemotherapy before a stem-cell transplant, as well as uncertainties about duration of effect.
FDA Approves Xolremdi for Ultra Rare Immune Disorder
April 29th 2024Xolremdi is the first therapy for WHIM syndrome, which can cause recurrent lung infections and papillomavirus-related warts. It’s available in two doses: 400 mg for an annual cost of $496,400 and 300 mg for an annual cost of $372,300.
FDA Approves Pfizer’s Gene Therapy Beqvez for Hemophilia
April 26th 2024Beqvez (fidanacogene elaparvovec) is priced at $3.5 million, which is on parity with Hemgenix, the first one-time therapy to treat adults with hemophilia B. Pfizer’s warranty will refund insurers and continue to provide coverage for patients if they change insurers.
