- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
EARLY and BENEFIT: Bosentan beneficial in patients with early PAH and in patients with inoperable disease
Patients with early-stage pulmonary arterial hypertension (PAH) and those with either inoperable chronic thromboembolic pulmonary hypertension (CTEPH) or inoperable or post-pulmonary endarterectomy pulmonary hypertension can benefit from treatment with bosentan, according to study results presented at the European Society of Cardiology Congress 2007 in Vienna, Austria.

Key Points
EARLY
In patients with early-stage PAH, the use of bosentan delayed disease progression as evidenced by a reduction in peripheral vascular resistance (PVR), a delay in time to clinical worsening, and a trend towards improved exercise capacity, said Nazzareno Galie, MD, head of the Pulmonary Hypertension Centre, University of Bologna, Institute of Cardiology, Italy. Dr Galie presented the results of the Endothelin Antagonist Trial in Mildly Symptomatic PAH Patients (EARLY).
The 6-month trial included 185 mildly symptomatic patients aged ≥12 years who were randomized to bosentan 62.5 mg BID for 4 weeks followed by 125 mg BID for the remainder of the trial (n=93)or placebo (n=92). To be eligible, patients had to be in functional class II and were required to have a 6-minute walking distance (6MWD) <80% of normal (or <500 m and associated with a Borg dyspnea index score of ≥2 points).
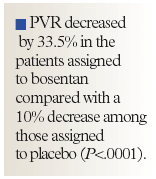
By the end of the study, PVR had decreased by 33.5% in the patients assigned to bosentan compared with a 10% decrease among those assigned to placebo (P<.0001).
The mean change from baseline in 6MWD was +11 m among bosentan recipients and –8 m among placebo recipients, representing a statistically nonsignificant treatment effect of 19 m (P=.0758).
The time to clinical worsening, defined as a composite of death, hospitalization for PAH, and symptomatic progression of PAH, occurred in 3% of patients who received bosentan and in 11% of patients who received placebo (P=.0114).
BENEFIT
The effect of bosentan in patients with CTEPH or post-pulmonary endarterectomy pulmonary hypertension was assessed in a subgroup analysis of the Bosentan Effects in Inoperable Forms of Chronic Thromboembolic Pulmonary Hypertension (BENEFIT) trial. Irene Lang, MD, Division of Cardiology, Medical University of Vienna, presented the study results.
For 10% to 50% of patients with PAH, there is an unacceptable operative risk for various reasons, including a mismatch between hemodynamic compromise and accessible occlusions, distal arteriopathy, and/or comorbidities. In addition, PAH may persist or reappear in 10% to 20% of surgical patients.
In this subgroup, the treatment effect of bosentan was assessed in 113 patients who had inoperable CTEPH and 44 who had previously undergone pulmonary endarterectomy. Patients in the study were randomized to bosentan 62.5 mg BID for 4 weeks followed by bosentan 125 mg BID for 12 weeks or to placebo (total treatment time, 16 wk).
Among the patients with persistent/recurrent pulmonary hypertension, treatment with bosentan was associated with a 32.5% greater reduction in PVR from baseline to Week 16 compared with placebo. There was a 17.5% greater reduction with bosentan treatment in patients with inoperable CTEPH compared with placebo.
There was no significant improvement in exercise capacity with use of bosentan or placebo in either group.
Patients with inoperable CTEPH experienced significant improvements in the Borg dyspnea index scores from baseline (treatment effect, –0.8), but the scores were not improved among patients with persistent/recurrent pulmonary hypertension who received bosentan (treatment effect, +0.1).
In both groups, bosentan was associated with improvements in mean cardiac index and mean N-terminal-pro-B-type natriuretic peptide.
Dr Lang said that the disconnect between the improvement in hemodynamic parameters and the lack of improvement in exercise capacity may be due to the short duration of the trial. She said that the prognosis in CTEPH is related to hemodynamics and that patients with inoperable disease may now have a medical option.
According to Dr Galie, "There have been multiple attempts to transfer the effects of targeted treatment for pulmonary arterial hypertension to inoperable patients or those not successfully operated on. This is the first time a randomized controlled study has shown favorable hemodynamic effects of a targeted therapy in this group of patients."
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
