- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Erythropoiesis-stimulating agents for anemia in older adults
Erythropoietin, a humoral factor produced predominantly in the kidney, stimulates red blood cell production in the bone marrow. Erythropoiesis-stimulating agents (ESAs) have been used for years in the treatment of anemia, with extensive experience and benefits in anemia of chronic kidney disease. Recent data have suggested adverse consequences with use of ESAs, perhaps relating to inappropriate use, and prompting release of guidelines to ensure safe use and maximize benefit. When prescribing ESAs, indications, requirements to monitor laboratory parameters (hemoglobin levels and ferrokinetics), and clinical status need to be stringently followed.

Key Points
Abstract
Erythropoietin, a humoral factor produced predominantly in the kidney, stimulates red blood cell production in the bone marrow. Erythropoiesis-stimulating agents (ESAs) have been used for years in the treatment of anemia, with extensive experience and benefits in anemia of chronic kidney disease. Recent data have suggested adverse consequences with use of ESAs, perhaps relating to inappropriate use, and prompting release of guidelines to ensure safe use and maximize benefit. When prescribing ESAs, indications, requirements to monitor laboratory parameters (hemoglobin levels and ferrokinetics), and clinical status need to be stringently followed. (Formulary. 2008;43:297–303.)
Erythropoiesis-stimulating agents (ESAs) have been used to treat anemia for nearly 2 decades; however, several developments have prompted FDA to issue new recommendations for their use. Although ESAs are effective in certain anemias, it is essential to use them appropriately. On November 8, 2007, FDA issued several warnings regarding their use in cancer and chronic kidney failure, essential knowledge for practicing physicians in all settings.
AGING, ANEMIA, AND ERYTHROPOIETIN
Anemia is defined by the World Health Organization (WHO) as a hemoglobin (Hb) level <12 g/dL for women and <13 g/dL for men.1 Although several criteria for anemia exist, the WHO criteria that were used in the National Health and Nutrition Examination Survey (NHANES) III are considered appropriate for older persons in spite of gender bias and other potential drawbacks. Based on this definition, the prevalence of anemia in the patients aged >65 years was 10.6%, prevalence increasing with age to >20% in patients aged ≥85 years.2 Anemia is linked to morbidity in the elderly. In ambulatory hospitalized elderly, the incidence of in-hospital falls was higher in patients with lower Hb level.3 Cardiac function in both coronary artery disease (CAD) and heart failure tends to worsen in presence of anemia; outcomes following treatment of anemia in heart failure are currently being studied.4,5 Older patients without anemia were reportedly healthier and encountered fewer hospitalizations than patients with anemia. Hb levels <11 g/dL in nursing home residents was associated with a lower 5-year survival rate.6 Community-dwelling elderly patients with anemia also manifest similar findings relating to mortality and hospitalization and have poorer functional and cognition status.7 These unfavorable outcomes in anemia emphasize the importance of anemia management and effect on outcome.
A randomized, double-blind trial in African-American women with chronic anemia demonstrated increased Hb during epoetin alfa treatment and improved quality of life.8 In particular, correction of anemia has been linked to favorable outcomes, including improved quality of life in anemia of CKD, based predominantly on the use of ESAs.8,9 Nevertheless, a full evaluation should be undertaken to determine etiology of anemia, even in CKD.10
If anemia is not a normal consequence of aging, what happens to erythropoietin levels with age? Erythropoietin levels do not change significantly with age; however, there may be resistance to the action of erythropoietin through interaction with pro-inflammatory cytokines (eg, interleukin-6).11 Further, levels of erythropoietin may vary based on cause of anemia such as iron deficiency, cancer, infection, and presence or absence of CKD. An inverse relationship exists between serum erythropoietin level and Hb concentration in elderly patients with iron deficiency anemia.12
AGING, KIDNEY FUNCTION, AND ERYTHROPOIETIN
A compensatory response to anemia and tissue hypoxia results in increased erythropoietin production from the specialized interstitial cells in the renal cortex. As with other organ function, age usually involves a functional decline in kidney function, beginning when patients are in their 30s with approximately a 1% annual decline in glomerular filtration rate on average. A decline in erythropoietin production by the kidneys often occurs when the creatinine clearance (CrCl) is ≤60 mL/min; however, a normal serum creatinine level is no guarantee that the erythropoietin-hypoxia feedback is intact. In a study of patients aged >65 years, consistently those with a CrCl <30 mL/min had lower endogenous erythropoietin levels and increasing anemia from decline in renal function; erythropoietin levels have been stratified to CrCl and Hb.12,13 From a functional perspective, the response of erythropoietin production to a decline in Hb levels is not impaired in healthy older patients. Further, erythropoietin levels are not obtained in evaluation of anemia in routine practice.
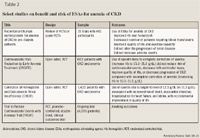
Anemia is common in CKD. The NHANES and Kidney Early Evaluation Program (KEEP) data suggest that anemia increases in prevalence in those aged ≥61 years with worsening CKD (stage 3 and higher).14 More than half of those aged >75 years with stage 3 CKD or higher are anemic.2,14 Although the predominant cause of anemia of CKD is relative deficiency of erythropoietin, any cause of anemia may be contributory (such as blood loss or iron deficiency) and a full evaluation is necessary.
THE USE OF ESAs IN ANEMIA



ESAs in perioperative setting. In a randomized, double-blind, multicenter study, epoetin alfa administered 4 weeks before total hip arthroplasty decreased need for allogeneic blood transfusion perioperatively.27 However, surgery procedures in elderly patients, especially invasive neurosurgery, total hip arthroplasty, major vascular surgery or radical cystectomy, increase the risk of deep vein thrombosis or pulmonary embolism postoperatively.28 ESAs also increase the rate of deep venous thromboses in patients undergoing surgery not on prophylactic anticoagulation. In February 2007, FDA received preliminary results of a multicenter, randomized study of epoetin alfa compared to standard care in adults undergoing elective spinal surgery; there was an increase of deep venous thrombosis frequency in the epoetin alfa group.23 Therefore, it may be prudent to provide prophylactic anticoagulation in this setting, in addition to nonpharmacologic measures.
ESAs for anemia in zidovudine-treated, HIV-infected patients. In a review of 4 clinical trials involving 435 patients with HIV (2 trials involved children and patients aged <65 y) and with anemia, ESAs reduced transfusion requirements, increased Hb levels, and improved quality of life in HIV-infected patients with anemia.29 The methodology in the randomized trials involved were questionable.29 Further, response to ESAs may be blunted in anemia of HIV infection.
RESISTANCE TO ACTION OF ESAs

KEY POINTS TO CONSIDER
Aging by itself seldom causes a decline in erythropoietin levels; alterations in production and response to erythropoietin are complex and influenced by the cause of anemia, kidney function, and presence of resistance. Recognizing the underlying cause of anemia is an appropriate initial approach, with use of ESAs for select situations. Once ESAs have been selected to treat a particular anemia, healthcare providers must remain appraised of developments, and follow approved indications and guidelines regarding its use. Providers must treat to target Hb/hematocrit levels and regularly monitor laboratory parameters to minimize adverse events. Used appropriately, ESAs can substantially improve the health status and quality of life of older adults.
EDITORS' NOTE: This article originally appeared in Formulary's sister publication Geriatrics (Dharmarajan TS, Widjaja D. Erythropoiesis-stimulating agents in anemia. Geriatrics. 2008;63:13–29).
Dr Dharmarajan is a professor of medicine, New York Medical College, Valhalla, New York; Chairman, Department of Medicine, and Chief, Division of Geriatrics, and Director, Geriatric Medicine Fellowship Program, Our Lady of Mercy Medical Center, Bronx, New York. Dr Widjaja is a fellow in geriatric medicine, Our Lady of Mercy Medical Center, and University Hospital of New York Medical College.
Disclosure Information: Dr Dharmarajan discloses that he has served in the past as a speaker for Ortho Biotech. Dr Widjaja reports no relationships with manufacturers.
REFERENCES
1. Nutritional anemias: Report of a WHO scientific group. Geneva, Switzerland 1968.
2. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: Evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268.
3. Dharmarajan TS, Avula S, Norkus EP. Anemia increases risk for falls in hospitalized older adults: An evaluation of falls in 362 hospitalized, ambulatory, long-term care, and community patients. J Am Med Dir Assoc. 2007;8(3 suppl 2):e9–e15.
4. Kosiborod M, Curtis JP, Wang Y, et al. Anemia and outcomes in patients with heart failure: a study from the National Heart Care Project. Arch Intern Med. 2005;165:2237–2244.
5. van Veldhuisen DJ, McMurray JJ. Are erythropoietin stimulating proteins safe and efficacious in heart failure? Why we need an adequately powered randomised outcome trial. Eur J Heart Fail. 2007;9:110–112.
6. Kikuchi M, Inagaki T, Shinagawa N. Five-year survival of older people with anemia: variation with hemoglobin concentration. J Am Geriatr Soc. 2001;49:1226–1228.
7. Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006;107:3841–3846.
8.Agnihotri P, Telfer M, Butt Z, et al. Chronic anemia and fatigue in elderly patients: results of a randomized, double-blind, placebo-controlled, crossover exploratory study with epoetin alfa. J Am Geriatr Soc. 2007;55:1557–1565.
9. KDOQI, National Kidney F. II. Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease in adults. Am J Kidney Dis. 2006;47(5 suppl 3):S16–85.
10. Balducci L. Epidemiology of anemia in the elderly: Information on diagnostic evaluation. J Am Geriatr Soc. 2003;51(3 suppl):S2–9.
11. Ershler WB. Biological interactions of aging and anemia: a focus on cytokines. J Am Geriatr Soc. 2003;51(3 suppl):S18–21.
12. Ble A, Fink JC, Woodman RC, et al. Renal function, erythropoietin, and anemia of older persons: The InCHIANTI study. Arch Intern Med. 2005;165:2222–2227.
13. Fehr T, Ammann P, Garzoni D, et al. Interpretation of erythropoietin levels in patients with various degrees of renal insufficiency and anemia. Kidney Int. 2004;66:1206–1211.
14. Brown WW, Peters RM, Ohmit SE, et al. Early detection of kidney disease in community settings: The Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2003;42:22–35.
15. Information on Erythropoiesis Stimulating Agents (ESA) (marketed as Procrit, Epogen and Aranesp). Prescribing information. US Food and Drug Administration Web site. http://www.fda.gov/cder/drug/infopage/RHE/default.htm. Accessed January 1, 2008.
16. Salmonson T, Danielson BG, Wikstrom B. The pharmacokinetics of recombinant human erythropoietin after intravenous and subcutaneous administration to healthy subjects. Br J Clin Pharmacol. 1990;29:709–713.
17. Heatherington AC, Schuller J, Mercer AJ. Pharmacokinetics of novel erythropoiesis stimulating protein (NESP) in cancer patients: Preliminary report. Br J Cancer. 2001;84(suppl 1):11–16.
18. Cody J, Daly C, Campbell M, et al. Recombinant human erythropoietin for chronic renal failure anaemia in pre-dialysis patients. Cochrane Database Syst Rev. 2001(4):CD003266.
19. Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084.
20. Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098.
21. Pfeffer MA. An ongoing study of anemia correction in chronic kidney disease. N Engl J Med. 2007;356:959–961.
22. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471–530.
23. Information on Erythropoiesis Stimulating Agents (ESA) (marketed as Procrit, Epogen, and Aranesp). Information for Healthcare Professionals. US Food and Drug Administration Web site. http://www.fda.gov/cder/drug/InfoSheets/HCP/RHE200711HCP.htm. Accessed November 24, 2007.
24. Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: Randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260.
25. Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: A survival study. J Clin Oncol. 2005;23:5960–5972.
26. Khuri FR. Weighing the hazards of erythropoiesis stimulation in patients with cancer. N Engl J Med. 2007;356:2445–2448.
27. Feagan BG, Wong CJ, Kirkley A, et al. Erythropoietin with iron supplementation to prevent allogeneic blood transfusion in total hip joint arthroplasty. A randomized, controlled trial. Ann Intern Med. 2000;133:845–854.
28. White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003;90:446–455.
29. Marti-Carvajal AJ, Sola I. Treatment for anemia in people with AIDS. Cochrane Database Syst Rev. 2007(1):CD004776.
30. Macdougall IC, Cooper AC. Erythropoietin resistance: The role of inflammation and pro-inflammatory cytokines. Nephrol Dial Transplant. 2002;17(suppl 11):39–43.
