- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Exenatide LAR: A sustained-release formulation of exenatide for the treatment of type 2 diabetes
Currently, exenatide is the only FDA-approved GLP-1 receptor agonist and its use is limited by the need for twice-daily injections. A long-acting release formulation of exenatide is being evaluated in clinical trials to assess effects on glucose control and patient quality of life.

Key Points
Abstract
Despite the availability of multiple classes of glucose-lowering agents to treat type 2 diabetes mellitus, glycemic control often remains inadequate, with hemoglobin (Hb) A1c values above the treatment goal of 7%. One newer class of agents, the glucagon-like peptide (GLP-1) receptor agonists, targets the incretin system to increase insulin secretion in response to glucose stimuli, while also inhibiting glucagon secretion, slowing gastric emptying and inducing satiety. Currently, exenatide is one of the few FDA-approved GLP-1 receptor agonists and its use is limited by the need for twice-daily injections. A long-acting release (LAR) formulation of exenatide is being evaluated in clinical trials to assess effects on glucose control and patient quality of life. Data from published clinical trials of exenatide LAR show that a dose of 2 mg given by subcutaneous injection once weekly is associated with reductions in HbA1c, fasting plasma glucose and body weight. When compared to twice-daily exenatide, the LAR formulation appears to offer slightly increased efficacy. Exenatide LAR has been well tolerated in phase 2 and 3 clinical trials. Mild-to-moderate nausea is the most commonly reported adverse drug event. Long-term safety and efficacy data have not yet been published and more information is needed before exenatide LAR finds its place in therapy. Currently, once-weekly exenatide LAR is awaiting approval by FDA. (Formulary. 2010;45:43-51.)
Diabetes mellitus has been described as an epidemic. It is a chronic, progressive disease that generally requires lifelong treatment. Approximately 6.6%, or 285 million people, between ages 20 and 79 years will have diabetes in 2010.1 This number is expected to rise by as much as 50% in the next 20 years in the absence of effective preventive programs.1 In 2006, diabetes was the 7th leading cause of death listed on death certificates in the United States.2 Long-standing uncontrolled diabetes is associated with microvascular complications such as neuropathy, nephropathy, and retinopathy as well as macrovascular complications, notably an increase in cardiovascular disease and associated events.2,3 Each of these contribute to the high economic burden of diabetes care in the United States, estimated in 2007 to be $174 billion in direct and indirect costs.2
Drugs that act on the incretin system, including agonists at the GLP-1 receptor (GLP-1R) and DPP-4 enzyme inhibitors were evaluated as treatments for diabetes because of their role in the regulation of insulin secretion.6-11 Endogenous GLP-1, together with glucose-dependent insulinotropic polypeptide (GIP), form a class of hormones known as incretins. These hormones are derived from the gut in response to oral glucose stimulation and account for up to 60% of the insulin secreted in reaction to nutrient stimuli.7 As GLP-1 secretion is decreased in type 2 diabetes, it was recognized as a target for novel drug therapy.8,9,12 In addition to the insulin-dependent insulinotropic effect, exogenously administered GLP-1 also exhibits inhibition of glucagon secretion, slowing of gastric emptying time, and induction of satiety that can lead to suppression of appetite, decreased food intake, and eventually weight loss.7-11 GLP-1 is rapidly degraded by DPP-4, a proteolytic enzyme, resulting in a half-life of about 1 to 2 minutes.7-10 Therapeutic approaches are aimed at potentiating GLP-1 activity, either by identifying GLP-1R agonists with improved resistance to degradation or inhibiting breakdown by the DPP-4 enzyme.7-10 Exendin-4, a molecule derived from the saliva of the Gila monster (Heloderma suspectum), has been identified as an agonist at the GLP-1R with greater affinity than GLP-1.7-11
Exenatide (Byetta, Amylin and Eli Lilly) is a synthetic version of the exendin-4 molecule. It is one of the few GLP-1R agonists (or incretin mimetic), approved by FDA.5 Liraglutide (Victoza, Novo Nordisk) is another GLP-1R agonist for the treatment of type 2 diabetes, and it was recently approved by FDA.5 Exenatide is approved for use in type 2 diabetes as an adjunct to diet and exercise to improve glycemic control in adults.13 It can be used as monotherapy or in combination with other antidiabetic agents.13 Currently exenatide is available in a 5-µg dose or 10-µg dose prefilled injectable pen (1.2 mL or 2.4 mL), each containing 60 doses.13 In most individuals, exenatide concentrations remain detectable for approximately 10 hours after the last dose is given.9,13 Exenatide is to be injected subcutaneously within 60 minutes prior to morning and evening meals (or the 2 largest meals of the day separated by at least 6 hours).13
Therapeutic efficacy for exenatide has been demonstrated by several controlled clinical trials, in which exenatide administered twice daily showed beneficial effects on glycemic control in patients with type 2 diabetes who were suboptimally controlled on none, one, or more than one oral or injectable antidiabetic medication (Table 1).14-20 The current dosage formulation requires twice-daily injections and does not provide continuous GLP-1R activation.21
To determine if continuous GLP-1R activation improves glycemic control, a sustained-release, subcutaneous injection of exenatide for the treatment of type 2 diabetes has been under development. This long-acting release (LAR) formulation is based on Medisorb technology and has been given once-weekly in clinical trials.22 It has been theorized that a long-acting exenatide formulation could improve glucose control, tolerability, and adherence over the twice-daily formulation.21 If the new formulation is approved, it would most likely replace its parent compound and improve treatment adherence in a population weighed down by multiple daily or weekly injections.23
CHEMISTRY AND PHARMACOLOGY
Exenatide is a GLP-1R agonist, which acts to increase insulin secretion postprandially in response to glucose stimulation. It belongs to a class of drugs known as incretin mimetics and is a synthetic version of exendin-4 from Gila monsters. It shares 53% homology to full-length naturally occurring GLP-1, but has resistance to degradation by DPP-4 due to replacement of alanine with glycine at the N-terminus of the 39-amino acid peptide.6,7 Naturally occurring GLP-1 is secreted from human enteroendocrine L cells from the distal ileum and colon in response to nutrient intake but is degraded within minutes by DPP-4, affording it no clinical utility.9 This minor substitution of alanine with glycine at the second position increases the half-life of the synthetic exendin-4 molecule to approximately 2.4 hours.13 Not only does exenatide increase insulin secretion but it also exhibits actions to suppress glucagon secretion, slow gastric emptying, and increase satiety.6,7
Exenatide LAR uses an extended-release drug delivery system, that allows the exenatide concentration to be sustained over time.22,24 Exenatide is encapsulated in microspheres made of poly (D,L lactic-co-glycolic acid), a biodegradable polymer, that breaks down over time and allows a controlled rate of drug delivery.21-24
PHARMACOKINETICS
With injections of exenatide 5 or 10 µg given twice daily, exenatide has been shown to reach median peak plasma concentrations in 2.1 hours and to have mean clearance of 9.1 L/h and half-life of 2.4 hours.13 Mean peak exenatide concentration was 211 pg/mL and overall area under the curve (AUC) increased proportionally from a dose of 5 µg to 10 µg. Absorption is similar when given subcutaneously in the abdomen, thigh or upper arm. The apparent volume of distribution of exenatide after a single injection of exenatide 10 µg is 28.3 L.13 Animal studies have shown that exenatide goes through glomerular filtration and is degraded by proteolytic enzymes.13,25
When exenatide LAR 2 mg was given once weekly by injection, the concentration reached 50 pg/mL by end of week 2.12,26 This level has been associated with reduced fasting and postprandial plasma glucose in previous studies using continuous infusion of exenatide.27 After 6 weeks of once-weekly injections, a steady-state concentration of 232 pg/mL was reached, which is comparable to the plasma exenatide concentration achieved with one single injection of exenatide 10 µg.12 After 15 weeks of exenatide 2 mg once-weekly treatment, no further injections were given and concentrations became subtherapeutic by week 21.12 Another study that tested exenatide LAR 2 mg once weekly reached mean plasma steady state concentrations of 71.7 pmol/L between weeks 6 and 10 and achieved a therapeutic plasma concentration in week 2.21
CLINICAL TRIALS
A 15-week, multicenter, randomized, placebo-controlled, double-blinded phase 2 study, compared exenatide LAR (0.8 mg or 2 mg) administered subcutaneously once-weekly.12 Patients in the placebo group were given injections containing 0.5% ammonium sulfate LAR instead of exenatide. Subjects had type 2 diabetes treated for at least 3 months, an HbA1c of 7.1% to 11% and were suboptimally controlled using metformin and/or diet and exercise. The primary outcomes were safety, tolerability and pharmacokinetics of exenatide LAR but the study also reported effects of the study drugs on glucose, HbA1c, and weight. Baseline patient (n=45) characteristics were: 40% female, mean ± SD of A1c 8.5%±1.2%, fasting plasma glucose (FPG) 9.9±2.3 mmol/L, weight 106±20 kg, and duration of diabetes 5±4 years. From baseline to week 15, exenatide LAR reduced mean ± SE HbA1c by -1.4%±0.3%% (0.8 mg) and -1.7% ±0.3%% (2 mg) compared to +0.4%±0.3% with placebo LAR, P<.0001 for both active treatments versus placebo. Additionally, an A1c ≤7% was achieved by 36% and 86% of study participants receiving 0.8- and 2-mg exenatide LAR, respectively, and 0% in the placebo group. FPG was lowered by 2.4±0.9 mmol/L (0.8 mg) and 2.2±5 mmol/L (2 mg) compared with an increase of 1.0±0.7 mmol/L in the placebo LAR group (P<.0001 for both active treatments vs placebo). In the exenatide LAR 2-mg treatment arm, body weight reductions of 3.8±1.4 kg were seen, while no change was noted in either the 0.8-mg exenatide LAR and placebo LAR arms. All results were clinically significant. No participants receiving exenatide withdrew from the study; adverse events reported included mild-to-moderate nausea, gastroenteritis and hypoglycemia.
To further evaluate exenatide LAR, a series of phase 3 clinical trials is under way.28 Results of the first trial: Diabetes therapy Utilization: Researching changes in A1c, weight and other factors Through Intervention with exenatide ONce weekly (DURATION) have been published. Interim results for DURATION-2 and -3 have been reported as abstracts. Clinical trials DURATION-4 and -5 are currently ongoing and DURATION-6 has not yet begun.29
The first DURATION study was published in October of 2008.21 DURATION-1 was a 30-week, randomized, open-label, non-inferiority study that compared exenatide LAR 2 mg given as a once-weekly dose with exenatide 10 µg given twice daily. Participants had to be at least 16 years of age and had a baseline A1c of 7.1% to 11%, FPG less than 16 mmol/L, a body-mass index (BMI) of 25 to 45 kg/m2. Patients also had to be weight-stable, defined as not fluctuating more than 10% for 6 months before screening. Patients could be drug naïve or currently treated with one or more oral diabetes medications including metformin, a sulfonylurea, or a thiazolidinedione, in combination with diet and exercise. All patients were started on 5 µg of exenatide given twice daily for 3 days prior to starting their assigned treatment arm. Participants were then given exenatide 2 mg once weekly by subcutaneous injection or exenatide 5 µg twice daily for 28 days followed by exenatide 10 µg twice daily for the remainder of the 30-week trial. The primary endpoint was mean change in HbA1c from baseline at 30 weeks.
The study enrolled 295 participants; 148 were randomly assigned to receive weekly exenatide and 147 to twice-weekly exenatide. Baseline characteristics were well matched across groups with about 47% female, HbA1c 8.3%±1 SD, mean FPG 9.2 to 9.6 mmol/L, and body weight 102 kg.
At the conclusion of 30 weeks, patients given exenatide LAR weekly had a change in HbA1c of -1.9 (SE 0.1%) compared with -1.5 (0.1%) in patients given twice-daily injections, a difference of 0.33% (P=.0023, 95% [confidence interval] CI -.54% to -0.12%). Changes in weight between both groups were similar, 76% of patients in the exenatide LAR treatment arm and 79% of patients in the twice-daily treatment group lost weight from baseline. Exenatide LAR resulted in a change from baseline of -3.7 (SE 0.5) kg compared with -3.6 (SE 0.5) kg in the exenatide twice-daily group. Change in FPG for the once-weekly treatment arm was -2.3 (SE 0.2) mmol/L vs -1.4 (SE 0.2) mmol/L for exenatide twice a day (P<.0001, 95% CI -1.3 to -.52) however, change from baseline for 2-hour post-prandial plasma glucose concentrations were greater in patients treated with exenatide 10 µg twice daily (-6.9 (SE 0.5) mmol/L compared to -5.3 (SE 0.5) mmol/L in the exenatide LAR treatment arm, 95% CI 0.4 to 2.9, P=.0124).
The most common adverse drug events (ADEs) from both treatment groups were mild nausea and vomiting and injection-site pruritus, with 6.1% of the exenatide once-weekly group and 4.8% of the twice-daily exenatide group withdrawing during the 30-week trial due to adverse events. Fewer patients in the LAR group reported nausea or vomiting than twice-daily group, although more LAR-treated patients reported injection-site pruritus.
After week 30, 258 of the original 295 patients from the DURATION-1 study opted into open-ended treatment with exenatide LAR. At the end of the original 30-week trial, patients receiving exenatide 10 µg twice a day were switched to exenatide LAR 2 mg weekly and those already taking exenatide LAR weekly continued receiving weekly injections. A 2008 abstract details effects on HbA1c, FPG, and weight from an additional 22 weeks of treatment.30 At week 52, reductions in HbA1c and FPG in patients initially treated with exenatide LAR were sustained: after 1 year, HbA1c was reduced by 2%±0.1% and FPG was reduced by 2.6±0.2 mmol/dL. In patients who switched to exenatide LAR at week 30 from twice-daily dosing, additional reductions in HbA1c and FPG were achieved with final results similar to once-weekly treated patients. At week 52, HbA1c was reduced by 2%±0.1% and FPG was reduced by 2.4±0.2 mmol/dL. It was reported that 75% of patients achieved HbA1c <7% and about 50% achieved HbA1c <6.5%. Weight loss was about 4 kg in both groups at week 52.
Treatment satisfaction and weight-related quality of life (QOL) were assessed in the original 30-week DURATION-1 study as well as in the open-ended, follow-up treatment.23 The Diabetes Treatment Satisfaction Questionnaire-status (DTSQ-s) and the Impact of Weight on Quality of Life-Lite (IWQOL-Lite) surveys were assessed at baseline and weeks 30 and 52. Statistically significant improvements for both treatment groups were seen between baseline and week 30, however treatment satisfaction and QOL were improved even more between weeks 30 and 52 for those patients who switched from twice daily dosing to once weekly dosing of exenatide.
Interim long-term data from the DURATION-1 study were presented at the annual meeting of the ADA in 2009.31 As described previously, patients from the DURATION-1 trial could opt into an open-ended treatment phase. The abstract details results from 131 patients who completed 2 years of treatment with exenatide LAR (exenatide 10 µg twice daily or exenatide LAR 2 mg once weekly for weeks 1-30, then 2 mg once weekly for all patients through week 102). A reduction in HbA1c of 1.8%±0.1% and a reduction of FPG of 37±4 mg/dL that was maintained after 2 years of treatment was reported. Body weight also was reduced by an average of 3.6±0.6 kg over 2 years of treatment. In addition, the study found total cholesterol, triglycerides and systolic blood pressure were reduced by 9.7±3.4 mg/dL, 18%, and 3.0±1.0 mm Hg, respectively. Nausea was the most commonly reported ADE in 8% of study participants over the 2-year treatment period.
Data from DURATION-2, a head-to-head study comparing exenatide LAR once weekly to sitagliptin or pioglitazone, were presented at a 2009 meeting of the ADA, but have yet to be published in full.32,33 DURATION-2 was a 26-week, double-blinded, superiority study in which 491 patients with type 2 diabetes suboptimally controlled using metformin were enrolled. Patients did not undergo a lead-in or a wash-out period. Treatment arms of the study include exenatide LAR 2 mg once weekly given by subcutaneous injection, sitagliptin 100 mg daily or pioglitazone 45 mg daily. A continuation of this study is ongoing with all patients continuing the trial after 26 weeks receiving exenatide LAR once-weekly. Preliminary results for exenatide LAR once-weekly, sitagliptin and pioglitazon, respectively were: HbA1c -1.55%, -0.92%, and -1.23% from baseline; and weight loss of 2.7 kg, 0.9 kg and gain of 3.2 kg. Approximately 80% of study participants finished the study and no major cases of hypoglycemia were noted in any treatment arm. The most common ADEs observed were nausea and diarrhea and specifically in the pioglitazone group, upper respiratory tract infection and peripheral edema.

DURATION-4 is currently under way and is evaluating patients with type 2 diabetes comparing exenatide LAR once weekly, metformin, sitagliptin, and pioglitazone.29 DURATION-5 is also actively ongoing, and is evaluating patients with type 2 diabetes comparing exenatide LAR once weekly to exenatide twice daily.29 These and other clinical trials are registered with the National Institutes of Health and can be found online at http://clinicaltrials.gov/.
Exenatide LAR has been evaluated in Japanese patients with type 2 diabetes.26 Previously published studies of exenatide LAR have included only patients from the United States and Canada. Iwamoto et al. assessed the safety, tolerability, pharmacokinetics, and pharmacodynamics of exenatide LAR once-weekly in a phase 1 randomized, placebo-controlled, double-blind, parallel study. Thirty Japanese patients were randomly assigned to receive exenatide LAR once weekly (0.8 mg or 2 mg) or placebo injections for 10 weeks. Eligible patients were age 20 to 75, weighed >50 kg, and had suboptimal HbA1c levels with diet and exercise alone or in combination with 1 or more antidiabetic agents including biguanides, sulfonylureas, or thiazolidinediones. Of note, suboptimal control was defined as levels between 6.5 and 9.5 or 10% depending on pre-study treatment regimen. HbA1c values <7% were considered controlled in other studies of exenatide LAR. The primary outcomes were safety and tolerability.
Baseline data were provided for 29 patients with evaluable date; n=10 in placebo group, n=10 in 0.8-mg exenatide LAR group, and n=9 in 2-mg exenatide group). Overall patients were 58.6% male, age 58±9 years with a BMI 26.3±2.9 kg/m2 and HbA1c 7.4%±0.8%.
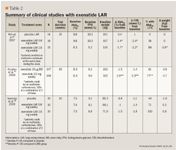
Results were similar to those from other published studies of exenatide LAR, with the exception of effects on weight.12,21 HbA1c was reduced from baseline by 0.4%±0.3%, 1.0%±0.7%, and 1.5%±0.7% for placebo, exenatide LAR 0.8-mg, and exenatide LAR 2-mg groups, respectively. FPG was also reduced by 20.5±20.4 mg/dL, 25.2±10.9 mg/dL, and 50.8±27.8 mg/dL for placebo, exenatide LAR 0.8-mg, and exenatide LAR 2-mg groups, respectively. Lastly, 2-hr postpandial glucose values were increased in placebo-treated patients by 8.8±26.9 mg/dL and reduced by 50.0±41.1, and 59.7±26.8 mg/dL (means±SD) in exenatide LAR 0.8-mg, and exenatide LAR 2-mg groups, respectively. Exenatide once-weekly had weight neutral effects in this population; those receiving exenatide LAR 2 mg losing only .05 kg by the end of follow-up. Of note, patients in this study had a BMI much lower than patients in North American studies (26-27 kg/m2 vs 35 kg/m2 ). Diabetes-related outcomes from published studies for exenatide LAR are summarized in Table 2.12,21,26
ADVERSE EFFECTS
In studies with exenatide LAR, once-weekly exenatide injections are well tolerated. Mild-to-moderate nausea is the most commonly reported ADE. In a phase 2 trial, nausea was reported by 15% of placebo-treated patients and 19% and 27% of participants in 0.8-mg and 2-mg exenatide-treated arms, respectively.12 Gastroenteritis was reported in 19% (0.8-mg dose) and 13% (2-mg dose) of study patients.12 Additional adverse events were minimal, including hypoglycemia and injection-site bruising. No patients withdrew from the study due to ADEs and no clinically significant abnormal vital signs; electrocardiogram (ECG) interpretations; or hematologic, chemistry or urinalysis values were recorded. By study end, 67% of patients had anti-exenatide antibodies, but these have no apparent effect on efficacy or safety of exenatide.12
In the 30-week DURATION-1 study, the most common adverse events among patients treated with 2-mg exenatide LAR once a week (n=148) were nausea (26.4%), injection-site pruritus (17.6%), diarrhea (13.5%), vomiting (10.8%), constipation (10.8%), and urinary tract infection (10.1%).21 A total of 9 subjects withdrew from the study due to adverse events. All hypoglycemia events were mild and occurred in patients concomitantly taking a sulfonylurea. No clinically significant abnormalities were seen with vital signs, ECGs, or in hematological, chemistry or urinalysis values. Anti-exenatide antibodies were detected in higher levels in patients receiving once-weekly dosing than twice daily, but the titers declined with time over the 30-week trial. Subgroup analysis showed patients with any level of anti-exenatide antibodies who were randomly assigned to once-weekly dosing had numerically greater HbA1c lowering than those treated with twice-daily dosing. Those with negative or low antibody titers appeared to have greater A1c lowering than those with high titers. It was concluded that the presence of antibodies did not correlate with adverse events.
In the short study of Japanese patients with type 2 diabetes, mild-to-moderate injection-site induration was the most common adverse event. Overall mild-to-moderate induration at the injection site, injection-site pruritis, nausea, and vomiting were associated more frequently with exenatide treatment than placebo.26 No serious ADEs were recorded and no patients withdrew from the study due to ADEs. Similar to previous studies, no major hypoglycemia events were seen. Similar to the other studies, 60% to 78% of exenatide LAR-treated patients developed anti-exenatide antibodies with no clinically relevant effect on HbA1c or hypoglycemia.
DRUG INTERACTIONS
Available clinical trials studying once-weekly dosing of exenatide LAR have not addressed the potential for drug interaction, although this may be clinically important as exenatide slows gastric emptying and may therefore affect drug absorption. Drug interactions with exenatide twice daily include acetaminophen, digoxin, lovastatin, lisinopril, oral contraceptives, and warfarin.13 In patients given acetaminophen elixir with or after exenatide 10 µg, both the peak plasma concentration (Cmax) and AUC were decreased, and the maximum plasma concentration (Tmax) was increased. If acetaminophen is given 1 hour before exenatide, the AUC, Cmax, and Tmax remain mostly unchanged. When exenatide 10 µg twice daily is given 30 minutes prior to digoxin doses, both the Cmax and Tmax are decreased, however the AUC of digoxin remains unchanged. In patients given exenatide twice-daily formulations (10 µg) 30 minutes prior to lovastatin administration, the AUC and Cmax were decreased and the Tmax was delayed. In clinical trials with patients on statins receiving exenatide 10 µg twice daily, this was not associated with changes in their lipid profiles from baseline. In patients currently taking lisinopril, exenatide twice-daily injections (10 µg) did not affect mean systolic and diastolic blood pressure or alter Cmax or AUC, but the Tmax was delayed by 2 hours. Oral contraceptives are recommended to be taken 1 hour prior to exenatide injections. Studies on patients using both warfarin and exenatide have shown no minimal alterations on the pharmacokinetic properties of warfarin; however the Tmax can be delayed by 2 hours if taken after repeated doses of exenatide.
DOSING AND ADMINISTRATION
At the present time, exenatide LAR has not been approved for use in the United States. Based on clinical trials in which exenatide LAR was given as 2 mg once weekly by subcutaneous injection, it is likely that this will be the approved dose and frequency for exenatide LAR.12,21,23,26 Treatment with exenatide LAR could have a lead-in period using exenatide 5 µg twice daily as seen in study populations.21 Exenatide twice daily is not recommended for use in patients with severe renal insufficiency or end-stage renal disease, defined as creatinine clearance less than 30 mL/min.25 Currently, published clinical trials of exenatide LAR have not included patients with significant renal insufficiency.
FORMULARY CONSIDERATIONS
Type 2 diabetes is a chronic, progressive disease that typically requires patients to monitor glucose levels and adhere to daily therapies, often for life. Published clinical practice guidelines offer recommendations for medical management of hyperglycemia. Patients should always be advised to use diet and exercise to control glucose and may also use medications, either alone or in combination. Preferred drug therapy options include metformin, sulfonylureas, and insulin; less-well validated options include various combinations of the preferred drugs plus pioglitazone or exenatide. Exenatide LAR is being studied as a treatment for type 2 diabetes and if approved, would likely replace twice-daily exenatide. There are extensive plans to produce supporting clinical data for exenatide LAR including 5 randomized, controlled trials and long-term follow-up. Although one 30-week study has been published in its entirety, most other clinical information has been released only in abstract form and 2 trials have not yet begun. Even twice-daily exenatide has only been available for a few years; data on long-term clinical and safety outcomes are not available. This is important because patients with diabetes may be on a particular drug for decades after diagnosis. Based on published data that have directly compared once-weekly to twice-daily exenatide, exenatide LAR appears to have greater glucose-lowering effects, while offering improved treatment satisfaction. After 30 weeks of treatment exenatide LAR appears to lower HbA1c by 1.5% to 2%, similar to metformin and sulfonylureas. The effect on HbA1c appears to be sustained after 2 years of treatment. By comparison, twice-daily exenatide was shown in clinical trials to lower HbA1c by about 1%. Both exenatide and exenatide LAR typically produce weight loss in North American patients, ranging from 1.6 to 3.8 kg.
Informed conclusions about the safety of exenatide LAR are premature, as only 3 short-term studies have been published in full. In those reports, exenatide LAR appeared to be well tolerated with adverse effects of the weekly formulation similar to those for the twice-daily formulation. No cases of pancreatitis have been reported in clinical trials. This medication will likely be used as maintenance therapy for years, but long-term safety data are not yet published.
Exenatide LAR compares favorably to twice-daily exenatide, with a reduced number of subcutaneous injections needed for a clinically relevant effect on HbA1c. One trial has specifically assessed treatment satisfaction and reported improvements in those patients using once-weekly exenatide LAR as compared to twice-daily exenatide. Information about how the drug will be made available to patients (eg, in a prefilled pen vs vial), storage requirements, and cost are not yet available. It would be expected that exenatide LAR will have similar precautions to the twice-daily formulation including warnings not to use the drug in patients with type 1 diabetes, renal insufficiency with creatinine clearance less than 30 mL/min, or those with a history of pancreatitis or severe gastrointestinal disease. Exenatide LAR is not available in any international market, although it has been studied in one group of Japanese patients.
If approved, exenatide LAR would likely replace twice-daily exenatide.
For the near-term, it is likely to remain a second- or third-line agent for the treatment of diabetes, primarily due to a lack of long-term safety data and data on important clinical outcomes such as microvascular or macrovascular complications.
Its use would likely be limited to patients with type 2 diabetes not controlled on sulfonylureas or metformin, who will agree to take a weekly subcutaneous injection and can afford therapy.
Ms Krause is a PharmD candidate, Northeastern University, Boston; Dr Kirwin is an associate clinical professor at Northeastern University, Boston.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, clinical manager, Department of Pharmacy Services, Hartford Hospital, Hartford, Conn, and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn; and by Craig I. Coleman, PharmD, associate professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
EDITORS' NOTE: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
REFERENCES
1. International Diabetes Federation. Diabetes e-Atlas. 4th ed. Prevalence of diabetes. Available at: http://atlas.idf-bxl.org/content/diabetes/. Accessed December 18, 2009.
2. Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Accessed December 18, 2009.
3. Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association; European Association for study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
4. Gryskiewicz KA, Coleman CI. Exenatide: a novel incretin mimetic hormone for the treatment of type 2 diabetes. Formulary. 2005;40:86-90.
5. Krentz AJ, Patel MB, Bailey CJ. New drugs for type 2 diabetes mellitus: what is their place in therapy?. Drugs. 2008;68:2131-2162.
6. Kolterman OG, Kim DD, Shen L, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health-Syst Pharm. 2005;62:173-181.
7. Chia CW, Egan JM. Role and development of GLP-1 receptor agonists in the management of diabetes. Diabetes Metab Syndr Obes. 2009;2:37-49.
8. Dungan K, Buse JB. Glucagon-like peptide 1-based therapies for type 2 diabetes: a focus on exenatide. Clin Diabetes. 2005;23:56-62.
9. Frias JP, Edelman SV. Incretins and their role in the management of diabetes. Curr Opin Endocrinol Diabetes Obes. 2007;14:269-276.
10. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696-1705.
11. Scheen AJ. Exenatide once weekly in type 2 diabetes. Lancet. 2008;372:1197-8.
12. Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30:1487-1493.
13. Byetta. [Package Insert]. San Diego, CA: Amylin Pharmaceuticals, Inc. 2009.
14. DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092-1100.
15. Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Exenatide-113 Clinical Study Group. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628-2635.
16. Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083-1091.
17. Zinman B, Hoogwerf BJ, Duran Garcia S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146:477-485.
18. Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143:559-569.
19. Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50:259-267.
20. Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naïve patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30:1448-1460.
21. 21. Drucker DJ, Buse JB, Taylor K, et al; DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomized, open-label, non-inferiority study. Lancet. 2008;372:1240-1250.
22. Press Release. Amylin, Lilly and Alkermes announce preliminary phase 2 results and submission of IND for exenatide LAR (sustained release) program in type 2 diabetes. San Diego, CA: Amylin Pharmaceuticals, Inc. 2003. Available at: http://investors.amylin.com/phoenix.zhtml?c=101911&p=irol-newsArticle&ID=392382&highlight=/. Accessed December 18, 2009.
23. Best JH, Boye KS, Rubin RR, Cao D, Kim TH, Peyrot M. Improved treatment satisfaction and weight-related quality of life with exenatide once weekly or twice daily. Diabet Med. 2009;26:722-728.
24. Medisorb Microspheres Technology. [Fact Sheet]. Cambridge, MA: Alkermes. 2009.
25. Linnebjerg H, Kothare PA, Park S, et al. Effect of renal impairment on the pharmacokinetics of exenatide. Br J Clin Pharmacol. 2007;64:317-327.
26. Iwamoto K, Nasu R, Yamamura A, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of exenatide once weekly in Japanese patients with type 2 diabetes. [advance publication]. Endocr J. 2009;56:951-962.
27. Taylor K, Kim D, Nielsen LL, Aisporna M, Baron AD, Fineman MS. Day-long subcutaneous infusion of exenatide lowers glycemia in patients with type 2 diabetes. Horm Metab Res. 2005;37:627-632.
28. Press Release. Plans for Exenatide Once Weekly NDA Submission by End of First Half of 2009 Reaffirmed Following FDA Feedback. Companies intend to use ongoing study to meet manufacturing comparability requirements. Indianapolis, IN: Eli Lilly, 2008. Available at: http://newsroom.Lilly.com/ReleaseDetail.cfm?ReleaseID=353958/. Accessed December 18, 2009.
29. Clinical Trials. Exenatide Once Weekly. Available at: http://clinicaltrials.gov/. Accessed December 18, 2009.
30. Buse J, Drucker D, Taylor K, et al. Exenatide once weekly elicits sustained glycemic control and weight loss over 52 weeks. Diabetologia. 2008;51:A146.
31. Kim T, Taylor K, Wilhelm K, Trautmann M, Zhuang D, Porter L. Exenatide Once Weekly Treatment Elicits Sustained Glycemic Control and Weight Loss over 2 Years. Presented at ADA 2009. San Diego, CA: Amylin Pharmaceuticals, 2009. Available at: http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=74462/. Accessed December 18, 2009.
32. Bergenstal R, Wysham C, Yan P, Macconell L, Malloy J, Porter L. DURATION-2: Exenatide once weekly demonstrated superior glycemic control and weight reduction compared to sitagliptin or pioglitazone after 26 weeks of treatment. Presented at ADA 2009. San Diego, CA: 2009. Available at: http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=74815/. Accessed December 18, 2009.
33. Press Release. Amylin, Lilly and Alkermes companies announce exenatide once weekly provided superior glucose control with weight loss compared to sitagliptin or pioglitazone in head-to-head DURATION-2 study. San Diego, CA: Amylin Pharmaceuticals, 2009. Available at: http://files.shareholder.com/downloads/LLY/785346901x0x283770/e53a7c32-2fa1-4516-b734-a1170001c18f/LLY_News_2009_3_31_Product.pdf. Accessed December 18, 2009.
34. Press Release. Amylin, Lilly and Alkermes companies announce exenatide once weekly provided superior glucose control compared to Lantus in head-to-head DURATION-3 study. San Diego, CA: Amylin Pharmaceuticals, 2009. Available at: http://newsroom.lilly.com/releasedetail.cfm?ReleaseID=397775/. Accessed December 18, 2009.
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
