- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Exenatide: A novel incretin mimetic hormone for the treatment of type 2 diabetes
A number of clinical approaches are utilized in managing the overlapping aspects of poor glycemic control in patients with type 2 diabetes. Exenatide (Amylin/Lilly), a novel drug in a new medication class known as the incretin mimetic agents, offers a new mechanism to achieve glycemic control.
Abstract A number of clinical approaches are utilized in managing the overlapping aspects of poor glycemic control in patients with type 2 diabetes. Exenatide (Amylin/Lilly), a novel drug in a new medication class known as the incretin mimetic agents, offers a new mechanism to achieve glycemic control. Through stimulation of the glucagon-like peptide receptor, exenatide stimulates the body's ability to produce insulin in response to elevated concentrations of blood glucose, inhibits the release of glucagon following meals, slows the rate of gastric emptying and secondarily the rate of nutrient absorption into the bloodstream, and reduces food intake. Clinical studies have demonstrated improved glycemic control with exenatide therapy in patients who are not using insulin and are not achieving target glucose levels with diet and oral medications. An NDA for exenatide was submitted to FDA in June 2004. (Formulary. 2005;40:86-90.)
An estimated 194 million adults worldwide have diabetes, including more than 18.2 million adults in the United States.1,2 In 2000, diabetes was the sixth-leading cause of death listed on US death certificates.2 Approximately 90% to 95% of those affected have type 2 diabetes.1 In type 2 diabetes, either the body does not produce enough insulin (ie, inadequate insulin secretion) or the cells ignore available insulin (ie, peripheral insulin resistance). The current approach to treatment is stepwise and systematic. Early in the approach, diabetic patients are encouraged to make healthy lifestyle modifications including changes in diet, exercise patterns, and weight control. For many patients, as glucose control continues to decline, pharmacologic treatment becomes necessary. Type 2 diabetes that is not controlled by lifestyle interventions and/or medications positions a patient for an increased risk for many serious complications, including cardiovascular disease, retinopathy, neuropathy, and nephropathy.2
Exenatide (Amylin/Lilly) is the first drug in a new class known as the incretin mimetic agents for the treatment of type 2 diabetes. It is initially being investigated to improve glucose control in patients who are not using insulin and are not achieving target glucose levels with diet and oral medications. An NDA for exenatide was submitted to FDA in June 2004.

PHARMACOKINETICS The pharmacokinetics of exenatide were evaluated in a multiple-dose study of 109 patients with type 2 diabetes.8 Following administration of a 0.08 μg/kg subcutaneous dose of exenatide, plasma exenatide levels increased steadily for all patients until reaching peak concentrations (Cmax) 2 to 3 hours after administration. Levels remained detectable for 6 hours post-dose. Disappearance from plasma was characterized by first-order pharmacokinetics.



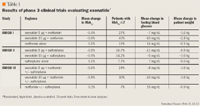
The AMIGO II study examined exenatide treatment versus placebo for a duration of 30 weeks in patients with type 2 diabetes not achieving target blood glucose levels with sulfonylurea therapy alone.12 In this triple-blind, multicenter study, 377 patients (60% men, aged 55±11 y, BMI 33±6 kg/m2, HbA1c 8.6±1.2% [±SD]) were randomized to treatment with the same dosing regimens employed in AMIGO I. All patients continued sulfonylurea therapy. As with AMIGO I, a greater proportion of exenatide-treated patients with baseline HbA1c >7% (n=353) achieved an HbA1c ≤7% compared to placebo. Reductions were observed in fasting plasma glucose in both exenatide arms compared with an increase in the placebo arm. Exenatide treatment groups had a greater mean change in body weight from baseline compared with placebo.

ADVERSE EFFECTS In studies, exenatide was generally well tolerated, with the most common adverse event reported being mild-to-moderate nausea, which occurred most frequently early in therapy. In the 30-week metformin-only AMIGO study,11 45% of patients taking 10 μg of exenatide reported nausea, compared to 23% for placebo. The drop-out rate due to nausea was 3% for 10 μg and 0% for placebo. In the metformin-sulfonylurea combination AMIGO study,13 49% of patients taking 10 μg reported nausea compared with 21% for placebo. The dropout rate due to nausea was 4% for 10 μg and <1% percent for placebo. Nausea appears to be minimized by gradual dose titration.8
Hypoglycemia rates were consistent with exenatide's glucose-dependent action, with no difference between the placebo and drug arms (5% for each group) in the metformin-only AMIGO study.11 In the metformin-sulfonylurea AMIGO III study,13 some patients taking exenatide in combination with sulfonylurea and metformin experienced mild-to-moderate hypoglycemia, with those taking the minimally effective doses of sulfonylureas reporting a lower incidence than those taking maximally effective doses. The incidence of mild-to-moderate hypoglycemia was 28% in the 10-μg exenatide group and 13% in the placebo group. One incident of severe hypoglycemia was reported in the 5-μg exenatide arm of this study. No participants withdrew from the study because of hypoglycemia.
Exenatide therapy has not been associated with weight gain.11,13 Exenatide has not been associated with clinically relevant adverse effects on heart rate, blood pressure, or the ECG in available studies assessing these parameters. Headache was relatively frequent with exenatide in one study.9 Small increases in cortisol levels have been reported on the first day of therapy with exenatide; levels typically normalize by day 28. This has also been observed with GLP-1 and does not appear to be of clinical relevance.
DRUG INTERACTIONS Currently there is no published information on drug interactions with exenatide. This may be related to clearance of exenatide and the fact that it does not appear to be cleared through the cytochrome P450 enzyme system.
DOSING AND ADMINISTRATION Exenatide had not received FDA approval at the time of publication. However, based on clinical trials, subcutaneous exenatide 5 μg twice-daily initially for 1 month, followed by 5 or 10 μg twice daily, has been effective in type 2 diabetes patients with poor glycemic control on metformin and/or sulfonylurea therapy.7,10-12
Dr Gryskiewicz is a clinical pharmacist at Hartford Hospital, Hartford, Conn. She can be reached at kgryskiewicz@harthosp.org
Dr Coleman is assistant professor of pharmacy practice, University of Connecticut School of Pharmacy, Storrs, Conn, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, director of Drug Information Services at Hartford Hospital, and associate clinical professor, University of Connecticut School of Pharmacy, and by Dr Coleman.
EDITORS' NOTE: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
REFERENCES 1. International Diabetes Federation. Diabetes e-Atlas. Prevalence: types 1 and 2 diabetes. Available at: http:// http://www.idf.org/e-atlas/home/index.cfm?node=53. Accessed February 15, 2005.
2. American Diabetes Association. National Diabetes Fact Sheet. Available at: http:// http://www.diabetes.org/diabetes-statistics/national-diabetes-fact-sheet.jsp. Accessed February 15, 2005.
3. Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. 1999 Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care. 1999;22:403-408.
4. Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;15;267:7402-7405.
5. Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regulatory Peptides. 2004;117: 77-88.
6. Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH sub 2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126-1131.
7. Fineman MS, Bicsak TA, Shen LZ, et al. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care. 2003;26:2370-2377.
8. Drucker DJ. Glucagon-like peptides. Diabetes. 1998;47:159-169.
9. Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003;88:3082-3089.
10. Kolterman OG, Kim DD, Shen L, Ruggles JA, Nielsen LL, Fineman MS, Baron AD. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health-Syst Pharm. 2005;62:173-181.
11. DeFronzo R, Ratner R, Han J, Kim D, Fineman M, Baron A. Effects of exenatide (synthetic exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes [abstract]. Presented at: American Diabetes Association 64th Annual Scientific Sessions; June 4-8, 2004; Orlando, Fla. Abstract 6-LB.
12. Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD, and the Exenatide-113 Clinical Study Group. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628-2635.
13. Kendall DM, Riddle MC, Dongliang Z, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight in patients with type 2 diabetes treated with metformin and a sulfonylurea. Presented at: American Diabetes Association 64th Annual Scientific Sessions; June 4-8, 2004; Orlando, Fla. Abstract 10-LB.
14. DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131: 281-303.
