- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
FDA and Novo Nordisk Warn about Counterfeit Ozempic
The FDA has seized thousands of units of counterfeit Ozempic 1 mg.
Lot and serial number for counterfeit Ozempic
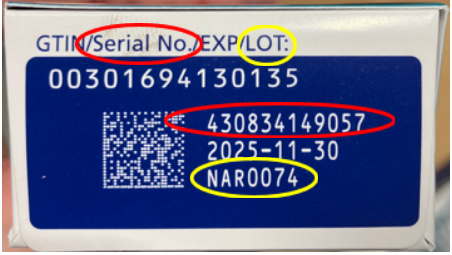
The FDA has seized thousands of units of counterfeit Ozempic (semaglutide) injection 1 mg. An analysis of the seized product has found the needles, pen label, accompanying information, and carton are counterfeit. The FDA and Novo Nordisk are testing the products for the drugs’ identify, safety and quality.
The products are labeled with lot number NAR0074 and serial number 430834149057.
The FDA is aware of five adverse events from this lot, although agency officials said in a press release that none of these events are serious, and they are consistent with known common adverse reactions to authentic Ozempic.
Novo Nordisk has a resource hub on safe use and on how to spot a counterfeit product of its semaglutide products, which include Ozempic, Wegovy and Rybelsus. Pharmacies are urged to only obtain semaglutide products through authorized distributors and patients are advised to purchase through pharmacies only.
Related: FDA Warns About Certain Compounded Semaglutide Products
This announcement follows one issued in June about some compounded semaglutide products that may not contain the same active ingredient as FDA-approved products.
Semaglutide products Ozempic and Wegovy continue to be on the FDA’s drug shortages list because of increased demand.
