- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Formulary Decision-makers update P&T policies following COX-2 inhibitors
Following the voluntary withdrawal of rofecoxib in September 2004 and the subsequent FDA-requested withdrawal of valdecoxib in April 2005, formulary decision-makers are considering options in navigating the changing pain management landscape. With one remaining COX-2 inhibitor available on the market (celecoxib [Celebrex, Pfizer]) and more cardiovascular (CV) risk data available for the class, the efficacy, adverse events, risk/benefit profiles, and costs of both COX-2-selective and nonselective NSAIDs are receiving more attention than ever.
Although COX-2 inhibitors were originally marketed as a pain management option for patients with gastrointestinal (GI) complications, agents in the class have been more broadly marketed and prescribed in recent years, according to Garret FitzGerald, MD, chair of the Department of Pharmacology and director of the Clinical Research Center and Center for Experimental Therapeutics at the University of Pennsylvania School of Medicine, Philadelphia. A reduction in GI risk, not a complete elimination of risk, was the first selling point of the COX-2 inhibitor class, Dr FitzGerald said.
However, the superiority of COX-2 inhibitors over traditional NSAIDs in terms of efficacy has never been documented. "There's never been an effort to demonstrate superior efficacy with COX-2s over other NSAIDs. There's no argument for that. However, we have compelling evidence that there is a clear cardiovascular signal that effects 2 to 3% of the population," Dr FitzGerald said.
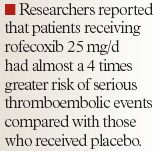
FDA acted on the committees' recommendations in April 2005 by asking that the manufacturers of all prescription NSAIDs, both COX-2-selective NSAIDs and nonselective NSAIDs, revise the labeling for their products to include a boxed warning highlighting the potential for increased CV events and any well-described, serious, potentially life-threatening gastrointestinal (GI) bleeding associated with their use.
FDA also asked Pfizer at that time to indefinitely suspend the sales and marketing of valdecoxib. According to FDA, this request was based on the potential increased risk for serious cardiovascular adverse events, an increased risk of serious skin reactions compared with other NSAIDs, and the fact that valdecoxib has not been shown to offer any unique advantages over other available NSAIDs.
With COX-2 and non-COX-2 NSAIDs presenting a potential CV risk for patients, formulary decision-makers have taken different approaches to the management of pain in managed care and hospital settings. Most prominently, the efficacy and potential GI benefits of COX-2-selective NSAIDs are being weighed against the cost and CV risk associated with the class.
Health plans across the country have implemented a number of measures to deal with the COX-2 inhibitor situation, including:

At Priority Health in Grand Rapids, Mich, a number of these measures have been implemented to manage the use of celecoxib among plan members. After the withdrawal of rofecoxib from the market and after FDA issued a Public Health Advisory regarding potential CV risk associated with prescription NSAIDs, plan members who had been prescribed a COX-2 inhibitor within the past 6 months and had any number of CV risk factors were identified and their physicians were contacted. The physicians received a letter identifying patients with CV risk factors and documented COX-2 inhibitor use and notified the physicians that the P&T committee would be reviewing the class in light of the recent CV risk data.
"What happened in September caused us pause," said Ed Keating, RPh, director of pharmacy, Priority Health, "and what happened in December made us take action."
Priority Health also stiffened PA requirements for the remaining COX-2 inhibitor, celecoxib. Essentially, celecoxib prescriptions are only approved for patients who are at risk for GI bleeding and who are not at risk for CV events, as indicated by the presence of hypertension, hypercholesteremia, congestive heart failure (CHF), diabetes, a family history of CV complications, or a previous myocardial infarction (MI). "We made it very robust," Mr Keating said.
As a result of the PA program and the withdrawal of rofecoxib and valdecoxib, Priority Health saw a significant decrease in the marketshare of COX-2 inhibitors among all NSAIDs. The COX-2 inhibitors boasted a 27% marketshare in September 2004 compared with a 14% marketshare in April 2005, according to Mr Keating.
Prior to the withdrawal of rofecoxib and valdecoxib, Independent Health of Buffalo, NY, covered celecoxib and rofecoxib in their preferred tier (tier 2) but used an electronic step therapy requiring trial and failure of traditional NSAIDs before a COX-2 inhibitor could be prescribed. The plan also required a split tablet of rofecoxib before allowing a full 25-mg tablet, and valdecoxib and meloxicam (Mobic, Boehringer Ingelheim) were placed in tier 3 with the same step therapy requirements.
With these measures already in place and in light of the actions of FDA, John Rodgers, RPh, MBA, director of pharmacy, Independent Health, said that the P&T committee at Independent Health sees little need for further action in the matter: "Since the FDA has taken action on the class to improve warnings and patient selection, I doubt we will take any further action and will likely leave Celebrex in tier 2 and Mobic in tier 3," he said.

The withdrawal of rofecoxib and valdecoxib has had little effect on the day-to-day operations of the Greenville Hospital System Pharmacy Services, Greenville, SC, according to Fred Bender, PharmD. According to Dr Bender, director of Pharmacy Services at Greenville Hospital System, there has been a slight increase in the prescribing of celecoxib, but no additional requests for other NSAIDs to be put on formulary. "Having a couple less COX-2s on the market has not had a significant impact on the formulary," he said.
Parkland Health and Hospital System in Dallas, Texas, is in a somewhat unique position with regard to the COX-2 inhibitor situation. One year prior to the market withdrawal of rofecoxib, a P&T subcommittee at Parkland performed an evidence-based assessment of the COX-2 inhibitor class and concluded that the drugs were no better in terms of efficacy and safety than traditional NSAIDs. The P&T committee subsequently removed the COX-2 inhibitors from the formulary.
"When we took the COX-2 inhibitors off formulary, we took off some popular drugs," said Vicki Crane, MBA, FASHP, RPh, vice president, Clinical Support Services, Parkland Health and Hospital System. "When you're paying dollars more per day and you're not getting any demonstrable benefits, it doesn't make sense," she said.
Many formulary decision-makers tend to agree that the risks associated with the COX-2 inhibitor class are not inherent; rather, the risk is more closely related to inappropriate use and overuse of these specialized NSAIDs. Patients should be dealt with on an individual level and more data are necessary to determine exactly what specific risk factors should be taken into consideration when prescribing COX-2-selective NSAIDs.
In order to make the most well-informed decisions regarding the COX-2 inhibitor class, Dr FitzGerald advised: "The safety and efficacy issues need to be reduced to the individual level instead of the population level."
FDA Approves Combination Therapy for Pulmonary Arterial Hypertension
March 25th 2024J&J’s Opsynvi is single-tablet combination of macitentan, an endothelin receptor antagonist, and tadalafil, a PDE5 inhibitor. It will be priced on parity with Opsumit, which is also a J&J product to treat patients with PAH.
FDA Issues Complete Response Letter for Onpattro in Heart Failure Indication
October 9th 2023Alnylam Pharmaceuticals will no longer pursue this indication of Onpattro and will instead on focus on a label expansion for Amvuttra, which is in phase 3 development to treat patients with cardiomyopathy of ATTR amyloidosis.
