- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
FUSION II: Nesiritide infusions in stage D HF not linked to increased risk of hospitalization, renal toxicity
Results of FUSION II, a randomized, double-blind, placebo-controlled study involving 920 patients with stage D chronic decompensated HF, presented at the 56th Annual Scientific Session of the ACC.

Key Points
Concerns about the renal safety of nesiritide arose after a meta-analysis and a large clinical trial (VMAC) associated the drug with a dose-dependent increase in serum creatinine, suggesting renal dysfunction. Previous clinical trials also resulted in a trend towards increased mortality at 30 days in nesiritide recipients. Important imbalances in baseline characteristics and background therapy among nesiritide recipients and recipients of its comparators prevented any firm conclusions about the effect of nesiritide on mortality when used according to its package insert.
The data from the new study, Follow-up Serial Infusions of Nesiritide in Advanced Heart Failure (FUSION II), offer reassurance in terms of nesiritide's renal safety and effect on mortality, as no excess renal toxicity or deaths were observed in patients randomized to nesiritide who also received aggressive background therapy.
FUSION II was a randomized, double-blind, placebo-controlled study involving 920 patients with stage D chronic decompensated HF. To be eligible, patients had to have ≥2 HF hospitalizations in the previous year, a left ventricular ejection fraction <40%, and New York Heart Association class IV symptoms or class III symptoms with creatinine clearance <60 mL/min.
Patients were randomized to once- or twice-weekly infusions of nesiritide, given as a 2-mcg/kg bolus followed by 0.01 mcg/kg/min (n=605), or placebo for 4 to 6 hours (n=306). These regimens were continued for 12 weeks followed by a 4-week taper and 8 weeks of follow-up.
All patients received intensive HF disease management throughout the study, which included optimal HF therapy and 1 or 2 outpatient clinic visits per week.
The primary end point was a composite of all-cause mortality and cardiorenal hospitalization at 12 weeks; this occurred in 36.7% of patients assigned to nesiritide and 36.8% of patients assigned to placebo (P=.79).
There were no significant differences in the incidence of all-cause mortality (9.5% in the nesiritide group and 9.6% in the placebo group; P=.98). The incidence of cardiorenal hospitalizations was 32.9% in the nesiritide group and 33.9% in the placebo group (P=.95).
The total event rate in FUSION II was approximately one-third lower than in the pilot FUSION I study, which Dr Yancy said was likely a result of the aggressive disease management in FUSION II.
In addition, the use of proven HF therapies (ie, ACE inhibitors or angiotensin-receptor blockers, diuretics, beta-blockers, aldosterone antagonists, nitrates) increased from baseline to the conclusion of the FUSION II study, which likely contributed to the lower event rate in this study, according to Dr Yancy.
The pilot study, FUSION I, included 210 patients with chronic HF who were at high risk for rehospitalization were randomized to serial infusions of nesiritide or placebo. At Week 12, there were no significant differences in the incidence of death or hospitalizations between the 2 groups.
The rate of adverse events was nearly equal in the 2 groups (88.7% among patients assigned to nesiritide and 86.9% among patients assigned to placebo; P=.45), but adverse events attributable to drug therapy were more frequent in the nesiritide group compared with the placebo group (42.0% vs 27.5%, respectively; P<.01). However, the incidence of adverse events leading to withdrawal of randomized therapy was not significantly different between groups (27.0% in the nesiritide group vs 25.5% in the placebo group; P=.63).
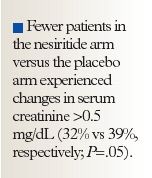
In response to questions about the excess of drug-related adverse events in the nesiritide-treated patients in FUSION I, Dr Yancy said: "I don't think these data are sufficient to declare that the drug is safe in all circumstances, but in the context of the way we studied it, we didn't see a signal of risk," referring to the lack of renal harm with nesiritide demonstrated in FUSION II; fewer patients in the nesiritide arm versus the placebo arm experienced changes in serum creatinine >0.5 mg/dL (32% vs 39%, respectively; P=.05).
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
