- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Generic EpiPen for kids may help with shortage
Teva’s generic EpiPen Jr. helps alleviate allergy drug shortage.
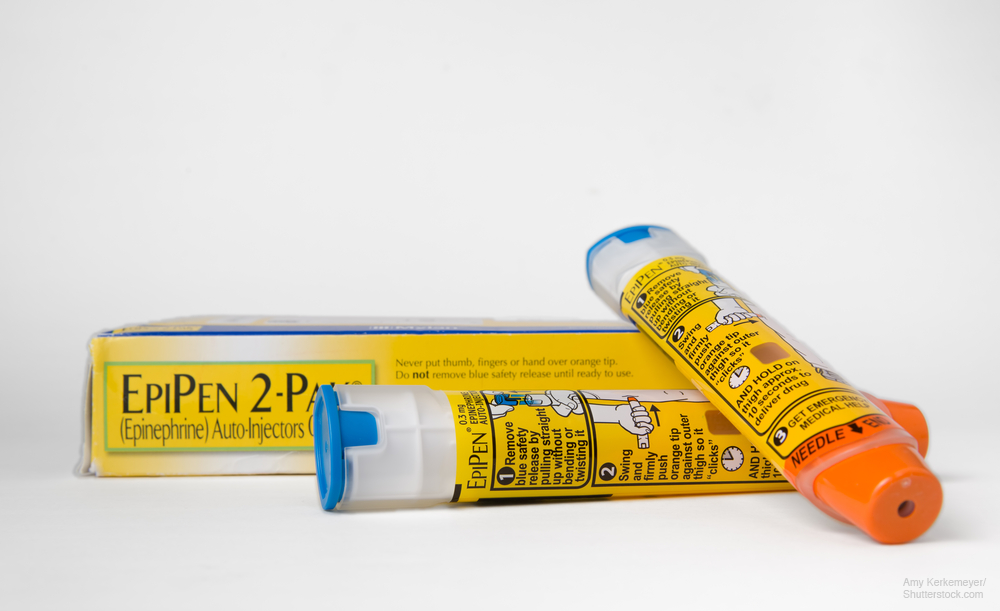
Soon after FDA approved generic versions of epinephrine injection USP (EpiPen, Mylan and Pfizer), a pharma maker is releasing a generic EpiPen for children.
Teva Pharmaceutical Industries said the FDA-approved version of EpiPen Jr. 0.15 mg is now available in most retail pharmacies. Teva’s generic version costs $300 per 2-pack.
Related: Epipen Shortage: Pharma Maker, Retailer Offer Aid
A year ago, FDA cleared the first generic version of epinephrine, granting Teva authority to make generic versions in 0.3 mg and 0.15 mg strengths.
“This approval means patients living with severe allergies who require constant access to life-saving epinephrine should have a lower-cost option, as well as another approved product to help protect against potential drug shortages.” said former FDA Commissioner Scott Gottlieb, MD, in a statement from FDA, at the time.
Related: Generic Epipen Poised To Alleviate Shortages
There is still a shortage of EpiPens in the U.S., Europe, and Canada. In fact, Pfizer and Mylan worked with FDA in June to extend the expiration dates by four months of all lots of EpiPen Auto-Injectors and its authorized generic version currently on the market in the US.
“Patients should have confidence in using the products as Pfizer works to stabilize supply,” Pfizer and Mylan said in a joint press release.
Meanwhile, Teva will continue working to ensure availability of both generic EpiPen strengths in the US and plan to accelerate production to meet the urgent need for this medicine,” said Brendan O’Grady, EVP and head of North America Commercial for Teva, in a statement.
Read more: FDA approves EpiPen rival
FDA Issues Complete Response Letter for Pz-Cel to Treat Epidermolysis Bullosa
April 22nd 2024Prademagene zamikeracel is a cell therapy designed to incorporate the functional collagen-producing COL7A1 gene into a patient’s own skin cells. The FDA is asking for additional information on manufacturing practices.
