- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
A growth hormone conversion program and patient outcomes at a not-for-profit HMO
Although experience tells us that formulary conversion programs are commonplace, they are discussed fairly infrequently in the literature. A recent MEDLINE search did not identify any conversion programs similar to a human growth hormone (HGH) program implemented at HIP Health Plan of New York (HIP-NY).
Abstract
Purpose: To evaluate current human growth hormone (HGH) product use within HIP Health Plan of New York (HIP-NY). To plan and implement conversion to 1 brand of HGH (Norditropin, Novo Nordisk) for all health plan HGH-utilizing members.
Methods: Communicate with HGH prescribers via letter and verbally. Provide prescribers with the necessary background information, educational tools, certificates of medical necessity (CMNs), and processes/procedures for obtaining Norditropin brand of HGH for all their current health plan HGH-utilizing members.
Conclusions: Due to the similarities in HGH products, HIP-NY was able to successfully implement a cost-effective HGH conversion program and maintain it, without any adverse clinical outcomes. (Formulary 2006;41:82–90.)
Although experience tells us that formulary conversion programs are commonplace, they are discussed fairly infrequently in the literature. A recent MEDLINE search did not identify any conversion programs similar to a human growth hormone (HGH) program implemented at HIP Health Plan of New York (HIP-NY). The most common conversion programs are for oral medications or similar medications for which there are many alternatives, or for well-known disease states (eg, hypercholesterolemia, hypertension, asthma, allergies, infections, etc) with predominantly oral therapies.1 However, conversion programs tend to be less prevalent when dealing with complex biotechnology and/or injectable drugs and certain diseases outside of the specialty pharmacy setting. Some of the disease states treated by these biotechnology drugs include multiple sclerosis, hepatitis C, or HGH deficiency-related disorders. These disease states can carry significant morbidity and mortality if not managed appropriately. Although treatment of growth hormone deficiency per se with HGH may not carry a significant risk of morbidity and mortality as some of these other conditions do, conversion to a different HGH product is still certainly a challenging undertaking.
Some of the difficulties that may be encountered (and that HIP-NY did encounter) in a HGH conversion program include:
A HGH conversion program can utilize a significant amount of resources and time. The desired outcome is optimal patient management that is appropriate and cost-effective without adverse clinical events. A program such as this would best be implemented in a setting in which resources are appropriately allocated. Resource allocation includes at least 1 clinical pharmacist who is knowledgeable in the main disorders that HGH is used to treat (eg, growth hormone deficiency, idiopathic short stature, small for gestational age). Whether this is in a managed care organization (MCO) with a large potential population, a small MCO, or a hospital/health system, as long as there are individuals who are committed to the project of converting HGH patients, similar results could be realized.
HIP-NY and its affiliates comprise a not-for-profit, mixed model health maintenance organization (HMO) that serves about 1.4 million members; it is the largest HMO in New York City by membership. HIP-NY provides medical care through contracted medical groups and through a network of more than 22,000 providers in more than 33,000 locations. Members may also see doctors in medical centers associated with leading hospitals and HIP-NY is affiliated with more than 100 participating hospitals. HIP-NY covers Medicare and commercial members and serves as its own pharmacy benefit manager (PBM). Twelve of the HMO's medical centers also have "in-house" pharmacies. The pharmacy staff in these pharmacies are employees of HIP-NY. The drug formulary is evaluated daily to ensure that it is up-to-date and reflective of current clinical evidence. HIP-NY's pharmacy and therapeutics (P&T) committee reviews all drugs, including HGH products, based on the current standards of practice. Since HIP-NY has managed its own formulary, 1 or more HGH products has been covered.

An in-house evaluation of the HGH products determined that these agents were similar regarding their safety and effectiveness. There were very limited reviews in the literature discussing this topic. Most of HIP-NY's key pediatric endocrinologists felt that the main difference between these agents was in the educational and administrative support provided by each individual HGH product's manufacturer. Two of HIP-NY's P&T specialty subcommittees recommended adding Norditropin as the preferred HGH product on the formulary because of ease of administra-tion/availability (eg, cartridges vs vials), the services to be provided by the manufacturer, and the cost. The services to be provided by the manufacturer (Novo Nordisk) at the time of the conversion were believed to be superior to the services that other manufacturers had provided or offered. The recommendation to add Norditropin as the preferred HGH product on the formulary was followed by a final approval by the P&T committee. As part of this recommendation to offer only 1 HGH product, both Humatrope and Nutropin/Nutropin AQ were removed from the formulary. Following the approval of the addition of Norditropin to the formulary, provider letters were prepared to be sent to current prescribers of HGH products informing them of the removal of Nutropin/Nutropin AQ and Humatrope from the formulary, and the addition of Norditropin. At this point, HIP-NY began planning and developing a HGH conversion program.
OBJECTIVE
The objective of this HGH conversion program was to convert patients from their current HGH product to Nordi-tropin. HIP-NY's market share for Humatrope in January 2004 before beginning the conversion program was 76%. For Nutropin/Nutropin AQ, the market share was 21% and other agent(s) market share was 3%. The Norditropin utilization and market share prior to the program was 0%. The patient mix was predominantly pediatric patients with growth hormone deficiency or idiopathic short stature (92%). All continuously enrolled patients were included. There were a few patients (n=8) who received Norditropin for off-label uses. These were approved by a plan medical director through HIP-NY's "non-FDA-approved use/dose" process. All of these patients received Norditropin therapy subsequent to the conversion program, in the continuation phase (essentially they were "new starts" on growth hormone treatment).
METHODS
At the end of 2003, a pharmacy utilization report was generated to determine how many patients were currently receiving HGH. Also identified were the HGH-treated patients' names, plan member identification numbers, the HGH brand name(s), the prescriber(s), the pharmacies where the HGH prescriptions were filled, and the last date filled.
TRANSITION PROCEDURE AND METHODS
In December 2003, the formulary deletion letters were sent to identified HGH prescribers informing them of the upcoming formulary changes. The letter asked them to start any new patients on the formulary product Norditropin and convert their current HGH-using patients to Norditropin. Prescribers were advised to convert patients to Nordi-tropin at their next office visit. Provisions were made for prescribers to continue with the current treatment (with a non-formulary HGH product) if the patient was not going to be seen in the office within the next 6 months. However, conversion was recommended shortly thereafter.
The conversion program began in January 2004 and was completed in June 2004. Along with the conversion to Norditropin, HIP-NY's certificate of medical necessity (CMN) was updated to promote the appropriate use of this agent. This CMN has been continually updated to reflect current practices and indications for HGH. As a component of the CMN, a prior approval (PA) requirement was implemented for all HGH therapies through the on-line pharmacy claims adjudication program (OCAP). A pharmacy block was also instituted for HGH therapies with a comment regarding the need for a prior authorization. In only 2 cases did the copayment force the conversion to the preferred HGH product from a non-preferred product. In these cases, the patients would have been responsible for 50% of the cost of the non-formulary product. Additionally, although the key pediatric endocrinologists and the specialty subcommittee members recommended the formulary changes, 1 of these individuals did not support the conversion once approved. None of this prescriber's patients were "converted to" or "started on" Norditropin. In only 1 case did that prescriber change a patient to Nordi-tropin. Overall, there did not appear to be a difference in the rate of change between the subcommittee members and non-committee members. Additionally, none of the plan prescribers were at risk for pharmacy benefit drugs, since the HGH products are covered under HIP-NY's pharmacy benefit.

Novo Nordisk sales support and clinical liaisons from ICORE Healthcare assisted with provider education and information aspects of the conversion program. Although ICORE Healthcare is a specialty pharmacy, the organization was not involved in the medication dispensing or patient education aspects of the conversion program. When physicians' offices were interested in receiving educational information about Norditropin, Norditropin SMN forms, and in-service training on the NordiPen Drug Delivery System, ICORE and the Novo Nordisk sales support and clinical liaisons assisted. This was coordinated by the pharmacy case manager and the Novo Nordisk managed care account executive. HIP-NY's corporate pharmacists also received in-service training focusing on proper NordiPen Drug Delivery System use and HIP-NY's conversion.
Some of the patient benefits of converting to Norditropin included receiving a free month of the drug; free in-home delivery of a backpack starter kit (including everything needed to get the patient started on Norditropin); and free clinical teaching on the NordiPen Drug Delivery System. This clinical training could be either in the doctor's office (if the patient and the doctor deemed this to be preferable) or through an "in-home" nursing visit coordinated through Nordicare. If the patient and their family opted for the "in-home" nursing visit, this was coordinated at the patient's convenience. If needed, a nurse fluent in a specific foreign language could be sent to the home. Nordicare also provided home patient education materials and physician office staff assistance. Some of the items in the backpack starter kit included: a Norditropin injection pen and storage case, a box of pen needles, a mini "sharps" container, a "how-to" video (in both VHS and DVD formats), a box of alcohol swabs, a cooler, and an ice pack (for storage).
As noted, the CMN, SMN and growth chart for each HGH-treated patient need to be provided to HIP-NY's Clinical Pharmacy Services Department. However, if the prescriber did not submit this paperwork prior to having the pharmacy adjudicate the HGH prescription, as noted, the pharmacy adjudication software blocks this and subsequent HGH prescription claims.
Once the forms (clinical documentation) and growth chart were received and evaluated by the pharmacy case manager, they were logged into a database. An approval for continuation or a denial was then issued. Approvals were issued by the pharmacy case manager and provided to the pharmacy for prescription adjudication and to the prescriber. Therapeutic denials were determined by a medical director, and a denial letter was sent to the patient and the provider. To coordinate mailing of the starter kit for Norditropin approvals, the SMN and clinical documentation were faxed to Nordicare from HIP-NY's Pharmacy Services Department.
RESULTS
Originally there were 16 different prescribers who wrote prescriptions for 26 different patients. Of these 16 prescribers, the majority of patients were from 4 different prescribers (or pediatric endocrinology groups) throughout the New York metropolitan area. Before the conversion program, no patients were receiving Norditropin. At the completion of the Norditropin conversion, the market share was 69% for Norditropin. At that time, some of the patients had not yet been seen by their physician, and some patients had filled their free month but had not yet filled their next Norditropin prescription. Therefore, the market share/pharmacy utilization of the plan would appear to be slightly lower that it truly was at that point in time. All the patients who converted to Norditropin remained on that HGH treatment.
Almost all of the converted patients opted for the in-home nursing visit (n=20), which was well-received. To accommodate a Spanish-speaking patient, a Spanish-speaking nurse was assigned to that case.

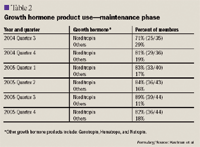
LIMITATIONS
Most of the potential challenges that were mentioned at the beginning of this article were encountered during this conversion program.
These included:
Once the prescriber was informed of the noted benefits of prescribing Norditropin, the educational aspects, the support and tools to be provided, and the agent's status on formulary, most prescribers converted their patients to Norditropin.
HIP-NY was able to sustain and improve the utilization of Norditropin over time.
Some of the limitations experienced during the maintenance phase are/were:
Other classes of biotechnology products that a conversion program similar to this could be applied to include: hepatitis C treatments (pegylated inter-feron/ribavirin products), tumor necrosis factor (TNF) blockers (eg, rheumatoid arthritis or other similar autoimmune conditions), or multiple sclerosis treatments (eg, interferon products).
CONCLUSIONS
Due to the similarities in HGH products, HIP-NY was able to successfully implement a cost-effective Norditropin conversion program, realizing a 27% cost savings in the growth hormone category of agents without any known adverse clinical outcomes. In 2005, the plan was able to increase its Norditropin utilization to between 82% and 89%. Since successful implementation of the Norditropin conversion program, 2 clinical pharmacists now coordinate the review of clinical documentation and maintain the Nordicare program to ensure continued appropriate management of patients requiring HGH treatment.
Dr Kaufman is project leader, Clinical Pharmacy Programs, HIP Health Plan of New York, New York, NY; and adjunct faculty at St. John's University College of Pharmacy, Jamaica, NY. She can be reached at mkaufman@hipusa.com
. Dr Alimonos Brodin was director, Clinical Pharmacy Programs, HIP Health Plan of New York, at the time the manuscript was prepared. Ms Sarafian is vice president, Pharmacy Services Department, HIP Health Plan of New York.
Disclosure Information: The authors report no relevant financial disclosures as related to this article.
REFERENCES
1. Taylor AJ, Grace K, Swiecki J, et al. Lipid-lowering efficacy, safety, and costs of a large-scale therapeutics formulary conversion program. Pharmacotherapy. 2001;21:1130–1139.
2. AACE Growth Hormone Task Force. American Association of Clinical Endocrinologists Medical Guidelines for Clinical Practice for Growth Hormone Use in Adults and Children-2003 Update. Endocrine Practice. 2003;9:64–76. Available at: http:// http://www.aace.com/clin/clinicalguidelines/hgh.pdf. Accessed January 30, 2006.
3. Parker KL, Schimmer BP. Pituitary hormones and their hypothalamic releasing factors. In: Hardman JG, Limbird LE, eds. Goodman & Gilman's The Pharmacologic Basis of Therapeutics. 10th edition. New York: McGraw-Hill; 2001.
4. Lee JM, Menon RK. Growth hormone for short stature children without a hormone deficiency: issues and practices. Contemp Pediatrics. 2005;22(10):46–53.
5. Genotropin [package insert]. Kalamazoo, Mich: Pharmacia & Upjohn Company; 2004.
6. Humatrope [package Insert]. Indianapolis, Ind: Eli Lilly and Company; 2005.
7. Norditropin [package insert]. Princeton, NJ: Novo Nordisk Pharmaceuticals Inc; 2004.
8. Nutropin/Nutropin AQ [package insert]. San Francisco, Calif: Genentech, Inc; 2005.
9. Saizen [package insert]. Rockland, Mass: Serono, Inc; 2005.
10. Serostim [package insert]. Rockland, Mass: Serono, Inc; 2004.
11. Zorbtive [package insert]. Rockland, Mass: Serono, Inc; 2004.
12. Drug Facts and Comparisons. Available at: http:// http://www.factsandcomparisons.com/efacts.asp. Accessed December 2005 (updated monthly).
13. Tev-Tropin [package insert]. Sellersville, Penn: Teva Pharmaceuticals USA; 2004.
14. First Data Bank Online. Available at: http:// http://www.firstdatabank.com/. Accessed January 6, 2006.
