- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Guideline-specific management of atrial fibrillation
Each pharmacologic management strategy for atrial fibrillation has limitations and more research is needed to determine which agents are equally effective, yet safer alternatives. Providing thromboprophylaxis to decrease risk of ischemic stroke is a well-validated approach. However, deciding between rhythm or rate control may not be as straightforward.
Atrial fibrillation (AF) is the most common sustained supraventricular tachyarrhythmia encountered in the primary care setting. It is defined by disorganized electrical activity in the atrium, resulting in conduction of irregular impulses in the ventricle.1–3 If AF is left untreated, a rapid ventricular rate often results.2 Many theories have been postulated to explain the pathophysiology of this disorganized electrical activity.2 While more research is needed to identify a precise mechanism, the automatic focus theory and multiple-wavelet hypothesis are widely accepted and are both discussed in the American College of Cardiology (ACC)/American Heart Association (AHA)/European Society of Cardiology (ESC) 2006 guidelines.2 Treatment to reduce the morbidity associated with AF aims to restore heart rate, convert abnormal rhythm back to normal sinus rhythm, and to decrease stroke risk with antithrombotic therapy.
Atrial fibrillation can occur in a normal heart, but most frequently occurs in the presence of heart disease. Cardiac conditions that predispose to AF include rheumatic mitral valve disease, coronary artery disease, congestive heart failure (CHF), and hypertension.4 The prevalence of AF increases with age, with an estimated prevalence of 2% in persons aged 60 to 69 years, 5% in those aged 70 to 79 years, and 9% in persons aged 80 to 89 years.5 Approximately 70% of individuals diagnosed with AF are between 65 and 85 years of age.2 Atrial fibrillation may be asymptomatic or patients may present with a wide range of symptoms including palpitations, dyspnea, lightheadedness, syncope, and/or generalized fatigue.5,6
The ACC/AHA/ESC guidelines classify AF into types depending upon the duration of the most frequent presentation. However, it should be noted that more than one type of AF can coexist in the same patient.2 New onset AF, or the first-detected episode, is classified as paroxysmal if it self terminates in fewer than 7 days. Two or more episodes of paroxysmal AF is termed “recurrent” and episodes lasting longer than 7 days are classified as persistent AF. Atrial fibrillation with duration of more than 1 year despite the use of therapeutic management strategies is classified as permanent. Patients experiencing any type of AF who are younger than 60 years and have no evidence of structural heart disease or cardiopulmonary disease, including hypertension, are often classified as having lone AF.2,5 Identifying the most frequent presentation of AF simplifies classification in patients experiencing numerous episodes and helps to identify the best treatment strategy.
There are three categories of treatment goals for AF: providing prophylactic therapy to prevent thromboembolism, maintaining normal ventricular rate, and/or correcting the rhythm disturbance. Combining treatment strategies to meet more than one goal is often necessary for improved outcomes.
Antithrombotic therapy
Prophylaxis for thromboembolism is vital because stroke secondary to thromboembolic mechanisms is the most significant morbidity in patients with AF.1,2 Atrial fibrillation increases the risk of stroke 4- to 5-fold across all age groups.6 Ischemic stroke associated with AF carries twice the risk of death compared with stroke from other causes.1 In addition, stroke resulting from AF tends to be more severe than stroke originating from carotid artery disease, and can lead to substantial mortality, morbidity, disability, and longer hospital stays.5,7,8 The rate of ischemic stroke among patients with AF averages 5% per year, which is 2 to 7 times higher than that seen in the general population.1,2 The incidence of stroke also rises with advancing age, with the highest incidence (36%) occurring in patients aged 80 to 89 years.2 Stroke incidence can vary markedly among patients with AF depending on other concomitant predisposing risk factors. Though the thromboembolic mechanisms in AF are multifactorial and intricate, arterial blood stagnation, endothelial dysfunction, and systemic hypercoagulability are all implicated.2,9
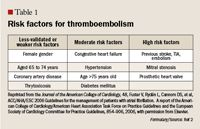
Table 1: Risk factors for thromboembolism
Many predisposing risk factors have been associated with increased stroke incidence in patients with AF.6 Several different stroke risk classification schemes have been developed to help identify patients’ stroke risk and to determine which patients will benefit the most from antithrombotic therapy.2 Risk factors associated with a highly increased stroke risk (>5% per year), moderately increased risk (3% to 5% per year), and lower risk are listed in Table 1.2 A simplified risk algorithm incorporating only the presence of CHF, hypertension, age >75 years, diabetes mellitus, and previous stroke (CHADS2) is also used and has been prospectively validated and statistically proven to better correlate stroke risk factors.2 Each CHADS2 risk factor carries 1 point except previous stroke or transient ischemic attack (TIA), which is associated with 2 points. Recommended antithrombotic therapy based on CHADS2 score is shown in Table 2 (page 158).2
Antithrombotic therapy is effective and should be given to all AF patients except those with lone AF or contraindications.1,6 However, the strength of evidence for the various antithrombotic therapies for stroke prevention is not equal.6 Several studies have compared the oral vitamin K antagonists (VKAs), such as warfarin, directly with aspirin therapy. These studies consistently show the inferiority of aspirin compared with VKAs for ischemic stroke prevention. A meta-analysis of 7 trials using an oral VKA versus aspirin found that the VKA was associated with a 46% greater reduction in ischemic stroke and a 36% greater relative reduction in all strokes than aspirin.6
Though warfarin is more effective than aspirin for stroke prevention, warfarin use may not be warranted in all patients. Several factors should guide the clinical decision when deciding between warfarin and aspirin to decrease thrombus formation and stroke risk. These factors include the CHADS2 index, risk of possible bleeding complications versus benefit of thromboprophylaxis, patient preference, and ease of monitoring.1,9 Generally, warfarin therapy should be reserved for patients with any high risk factor or more than one moderate risk factor for future stroke.6 For patients presenting with AF and one moderate risk factor, guidelines recommend warfarin rather than aspirin therapy.6 Though aspirin is significantly less effective than warfarin, it provides adequate protection for AF patients with no additional stroke risk factors (Table 2, page 158).2,6,9 Though evidence supporting aspirin has not been definitive enough to recommend a specific dose, current guidelines suggest lower doses ranging from 75 mg to 100 mg per day as the best balance between efficacy and safety.6

Table 2: CHADS2a risk index and recommended antithrombotic therapy
Although effective, warfarin treatment is associated with increased risk of bleeding, numerous medication and food interactions, and a life-long commitment to laboratory monitoring. Patients treated with warfarin should maintain an international normalized ratio (INR) between 2 and 3, specifically 2.5.6 This narrow range of anticoagulation is associated with optimal stroke protection and a minimal risk of major hemorrhage.2 At INR values 2 At INR values >4, the risk of intracranial hemorrhage increases sharply. For patients >75 years of age at increased risk for bleeding but without a contraindication to anticoagulation, or for patients unable to tolerate full-dose anticoagulation, an INR range of 1.6 to 2.5 may be considered.2,6 However, this strategy is controversial because an INR 6
Rate versus rhythm control
While the clinical benefit of thromboembolism prevention is consistent and well-validated, the decision to cardiovert to normal sinus rhythm or maintain rate control is not as straightforward. For some patient populations a combination of rhythm and rate control may be the best strategy. Two landmark clinical trials comparing rhythm control to rate control have shown that rhythm control is not superior to rate control.6 The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) and Rate Control Versus Electrical Cardioversion for Persistent Atrial Fibrillation (RACE) were the 2 large landmark trials.10,11 Results of AFFIRM showed no significant difference between rate control and rhythm control for the primary end point of overall mortality (P=.08) but researchers reported potential advantages for a rate control strategy, primarily fewer drug-related adverse effects.10 In the RACE trial, 39% of patients receiving rhythm control and 10% of patients receiving rate control achieved sinus rhythm but there was only a 0.4% difference in mortality and morbidity between the 2 groups.11
Initial and ongoing AF management should be individualized for each patient. Results from the AFFIRM and RACE trials favoring rate control to achieve sinus rhythm pertain mostly to patients aged 68 to 70 years who are asymptomatic.6 For younger patients or highly symptomatic patients without underlying heart disease, rhythm control may be the preferred strategy.4,6
The National Institute for Health and Clinical Excellence (NICE) guideline for AF recommends rhythm control for asymptomatic patients 12,13 In contrast, results of a recent clinical trial including CHF patients with left ventricular ejection fractions (LVEF) 13
Rate control
Sustained, uncontrolled tachycardia can cause deterioration of ventricular function leading to tachycardia-induced cardiomyopathy. Adequate pharmacologic rate control can generally reverse any deleterious effects.2 Definitions for appropriate rate control differ between clinical trials and with regard to patient age.2 However, maintaining heart rate between 60 to 80 beats per minute (bpm) at rest and 90 to 115 bpm during exercise is an accepted goal.14

Table 3: Rate control during atrial fibrillation
Beta-blockers, nondihydropyridine calcium channel blockers, digoxin, and in specific cases, amiodarone or dronedarone, are effective as monotherapy or in combination for rate control. The overall efficacy of pharmacologic agents for achieving ventricular rate control in AF clinical trials has been about 80%.2
The choice of agents for rate control is dependent upon specific idiosyncrasies of each medication; heart rate; the coexistence of heart failure, ischemia or left ventricular dysfunction (LVD); the presence of a pre-excitation syndrome; and specific patient characteristics.2,4 Evidence from the AFFIRM trial showed that beta-blockers were the most effective drug class for rate control.2,10 As a rule, digoxin is less effective than beta-blockers or calcium channel blockers at maintaining resting heart rate.4 For exercise-induced tachycardia, digoxin has not been shown to be effective.4 Amiodarone is effective for controlling ventricular rate in patients with AF, and is an alternative when calcium channel blockers, beta-blockers, and digoxin are ineffective. The potential toxicities of this agent prevent its routine use despite its effectiveness. Dronedarone, an amiodarone derivative with the iodine moieties removed to lessen the adverse effects seen with amiodarone, is effective but carries a black box warning and is contraindicated in patients with CHF. Table 3 (page 159) lists rate control agents with conditions in which their use is preferred or cautioned.2,4,8,29
Rhythm control
Pharmacologic agents can be used to restore sinus rhythm (cardioversion), maintain sinus rhythm, or both. The benefits for attempting to restore and maintain sinus rhythm with antiarrhythmic medications and/or synchronized external shocks are numerous and include improved left ventricular (LV) response, restoration of atrial systolic function, reduced heart rate and avoidance of tachycardia-induced cardiomyopathy, relief of symptoms and improved exercise tolerance, and improved quality of life.2,14 External shocks, or direct-current cardioversion (DCC), are associated with a higher success rate (80% to 90%) than pharmacologic cardioversion.5 The ACC/AHA/ESC guidelines provide recommendations for when DCC is appropriate.2,5 Although DCC is more effective than pharmacologic cardioversion, routine use is limited because of the need for conscious sedation or anesthesia.2 Antiarrhythmic medications are not without a separate host of limitations, and all antiarrhythmic medications are proarrhythmic and may aggravate or cause torsades de pointes (TDP) or other arrhythmias.
The main goal of antiarrhythmic drug therapy is to quickly relieve symptoms by shortening the time to conversion to sinus rhythm. The decision of which agent to use is dependent first on the duration of the arrhythmia.15 The literature provides insight into the best medications for recent-onset (7 days).16 Pharmacologic cardioversion seems to be most effective when initiated within 7 days of the arrhythmia occurring. Patients with a duration of AF 4 Dofetilide, flecainide, ibutilide, propafenone, and amiodarone are associated with the strongest efficacy when initiated within the first 7 days of arrhythmia onset.2 Less-effective or incompletely studied agents are disopyramide, procainamide, and quinidine.2 The efficacy of oral agents is greatly reduced after the first 7 days of arrhythmia onset, and DCC may be preferred.2,4 Dofetilide, amiodarone, and ibutilide are associated with the strongest efficacy data beyond 7 days.2
After determining arrhythmia duration, the presence or absence of cardiac structural damage, substantial left ventricular hypertrophy (LVH), ischemic heart disease, heart failure, and hypertension should be considered when choosing an agent.2,17 Specific idiosyncrasies of medications, consideration for ease of administration, and drug side-effect profiles are also equally important in the decision-making process. Many clinicians may opt for a rate control strategy over a rhythm strategy to avoid the risk of proarrhythmia such as TDP, sudden cardiac death, and other adverse events associated with antiarrhythmic agents.17
Vaughn Williams class type III antiarrythmics
Amiodarone lengthens the cardiac action potential and blocks myocardial potassium channels leading to slowed conduction and prolonged refractoriness.18 In addition to its class III characteristics, amiodarone also possesses qualities similar to other classes within the Vaughn Williams classification scheme. Efficacy of amiodarone depends on route of administration and the duration of the arrhythmia.19 Important electrophysiologic differences exist between oral and intravenous formulations making interpretation of the efficacy data confusing.2,20 While both formulations have similar effects on the atrioventricular (AV) node, oral amiodarone has a more pronounced effect on many other variables including prolongation of the action potential and increasing the ventricular refractory period.20
In recent onset AF, amiodarone does not appear to be any more effective than other agents with proven efficacy for restoring sinus rhythm.2 Amiodarone is considered a reasonable option as evidenced by its class IIa recommendation category indicating that dofetilide, flecainide, ibutilide, and propafenone-all with class I recommendation, strength A evidence status-should be considered first (Table 4).2,17 Amiodarone is preferred for patients with structural heart disease including LVD, and for patients with a contraindication to Vaughn Williams class IC agents. In many meta-analyses comparing amiodarone to placebo or class IC agents for recent onset AF, amiodarone was inferior at times up to 8 hours, but more effective than placebo and equally effective as class IC agents at 24 hours.21 Therefore, it is important to consider that the onset of action may be delayed, depending on route of administration, resulting in delayed cardioversion (Table 4, page 160).2,17 Predictors of amiodarone efficacy include shorter AF duration, smaller left atrial size, and higher dose.2
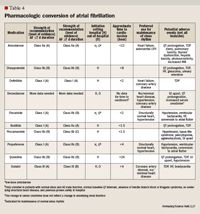
Table 4: Pharmacologic conversion of atrial fibrillation
Amiodarone is considered more effective than type I agents, sotalol, or placebo for long-term maintenance of sinus rhythm in patients with paroxysmal or persistent AF refractory to other antiarrhythmic medications.22 It is considered the most effective drug in maintaining sinus rhythm in patients with structural heart disease.22 However, because of its unfavorable side-effect profile, the lowest effective dose should be used to decrease the prevalence of the side effects.
Advantages of using amiodarone include consistently proven efficacy for maintenance of sinus rhythm, effective rate control, very low incidence of proarrhythmia, and little inotropic activity, making it an attractive choice for patients with heart failure, LVH, coronary artery disease, and/or previous myocardial infarction (MI).2,22 Disadvantages contributing to oral amiodarone not being a first-line choice include pulmonary fibrosis, thyroid abnormalities, elevation of transaminases, hypotension, bradycardia, visual disturbances, and warfarin-dosing complications. Similar to dofetilide and ibutilide, amiodarone causes QT interval prolongation.2,4,17
Dofetilide prolongs the action potential duration and increases myocardial refractoriness.18 There is sufficient evidence for dofetilide as a first-line choice for converting and maintaining normal sinus rhythm in patients with persistent AF.2 The Symptomatic Atrial Fibrillation Investigative Research on Dofetilide (SAFIRE-D) and the Danish Investigations on Arrhythmia and Mortality in Dofetilide (DIAMOND) trials were large-scale studies demonstrating the efficacy of this agent.23,24 SAFIRE-D showed dofetilide to be significantly more effective in maintaining sinus rhythm after 1 year compared with placebo, and the DIAMOND trials showed safety and efficacy in high-risk patients with CHF and previous MI. Like amiodarone, dofetilide does not increase morbidity or mortality in patients with CHF or LVD.23,24
There are risks associated with initiating dofetilide, highlighting a major difference between this agent and amiodarone. As outlined in the black box warning, clinicians and institutions are required to receive appropriate dosing instructions before dofetilide can be prescribed, dispensed, administered, or initiated. Initiation can only be attempted on an inpatient basis and patients must have a minimum 72-hour observation period. Creatinine clearance calculations and electrocardiographic monitoring must be performed.25 These strict precautions are due to the risk of life-threatening arrhythmias during initiation. Dosing dofetilide based on renal function and degree of QT prolongation results in a smaller risk of TDP, and clinical trial data show an overall incidence of TDP ranging from 0.9% to 3.3% with dofetilide use.26 Compared to dofetilide, amiodarone has less proarrhythmic effect and is associated with a TDP incidence of 26 The risk of TDP with dofetilide is increased during the first few days of initiation and has been associated with higher doses and electrolyte imbalances.18,27 Unlike amiodarone, dofetilide appears to be free of noncardiac side effects and is less likely to interfere with the effects of warfarin.26
Dronedarone was approved by FDA on July 2, 2009, and is the first agent in 10 years to be added to the armamentarium of anti-arrhythmics. It is indicated to reduce cardiovascular hospitalizations in patients with paroxysmal or persistent atrial flutter who are already in sinus rhythm or who will be cardioverted.28 This agent shares many similarities to amiodarone including antiadrenergic and electrophysiologic properties, QT interval prolongation, a low incidence of TDP, inhibition of P-glycoprotein, and inhibition of the CYP450 CYP3A and 2D6 isoenzymes.27,29,30 Because of clinically significant drug interactions, dronedarone’s use with potent CYP3A inhibitors and inducers should be avoided.28 Use of dronedarone with negative chronotropic agents such as beta-blockers and nondihydropyridine calcium channel blockers necessitates the need for low-dose initiation of the latter to prevent bradycardia and heart block. An empiric decrease in digoxin dose should be considered due to dronedarone’s inhibition of P-glycoprotein. Unlike amiodarone, dronedarone is a noniodinated compound with the addition of a methane sulfonyl group. These molecular changes should make dronedarone devoid of amiodarone’s iodine-related organ toxicity and decreased lipophilicity leading to a shortened half-life and less tissue toxicity.27,28,30,31 Dronedarone, unlike amiodarone, has not been associated with any significant changes in INR.
Trials of dronedarone versus placebo in patients with AF or atrial flutter show dronedarone to be more effective in maintaining sinus rhythm and in controlling ventricular rate during AF recurrences.32 Differences between study populations and outcomes in the Effect of Dronedarone on Cardiovascular Events in Atrial Fibrillation (ATHENA) trial and the The Antiarrhythmic Trial with Dronedarone in Moderate-to-Severe Congestive Heart Failure Evaluating Morbidity Decrease (ANDROMEDA) trial formed controversy about dronedarone’s role in patients with heart failure. The ATHENA trial showed a 24% reduction in the primary end point of death from any cause or cardiovascular hospitalization but excluded patients who had either hemodynamic instability or severe systolic heart failure.28,33 The ANDROMEDA trial failed to show promising results and was stopped prematurely due to an increased mortality risk for patients on dronedarone.34 In contrast to the ATHENA trial, ANDROMEDA included patients with symptomatic moderate-to-severe systolic heart failure with recent decompensation.34 Because of the ANDROMEDA results, a black box warning exists for dronedarone contraindicating its use in patients with New York Heart Association (NYHA) IV heart failure or NYHA II or III heart failure with recent decompensation requiring hospitalization or referral to a specialized heart failure clinic.28 Because of the ATHENA trial, dronedarone received the majority vote for approval from FDA for its current indication.
Direct comparisons between dronedarone and amiodarone are limited, but results from a meta-analysis of 4 trials comparing amiodarone with placebo and 1 unpublished manufacturer’s trial comparing dronedarone with amiodarone showed significant reduction in recurrent AF with amiodarone, but not dronedarone versus placebo. Amiodarone was superior to dronedarone in preventing recurrent AF (PP35 These results suggest that dronedarone is a safer alternative, but not a more effective alternative to amiodarone.
Controversy concerning dronedarone’s use continues. On March 30, 2010, NICE issued a second draft guidance reversing an initial guidance stating that dronedarone should not be used for treatment of AF. In the reversal under consideration, NICE would allow limited dronedarone use as a second-line agent in patients with uncontrolled, nonpermanent AF and at least one other cardiovascular risk factor.36 More recently, a review published in the April 13, 2010, issue of Journal of the American College of Cardiology concluded that dronedarone only be considered as a second- or third-line AF therapy. However, the article was accompanied by a commentary suggesting it as a first-line therapy because of its safety record compared with other available agents.37,38
Additional research is needed directly comparing dronedarone to other antiarrhythmics before its role in the management of AF can be determined.
Ibutilide is only available as an intravenous formulation and its use is limited to conversion for recent onset atrial fibrillation. Ibutilide’s efficacy for AF that persists longer than 90 days has not been well determined.2,39 It is an acceptable evidence-based strategy to use type IC antiarrhythmic agents after initial intravenous ibutilide to maintain sinus rhythm because type IC agents do not further prolong the QT interval.16 As discussed in the ACC/AHA/ESC practice guidelines, ibutilide can also be used as second-line treatment in patients who fail to convert to sinus rhythm following treatment with propafenone, or in those with recurrent arrhythmias receiving concomitant propafenone or flecainide. Some comparative studies have suggested ibutilide may be more effective than the type IA agent procainamide for cardioversion in recent onset AF.39
Like other antiarrhythmics, ibutilide can cause TDP and patients should be monitored with ECG for at least 4 hours after administration or until the QT interval has returned to baseline.16,39 This agent differs from other agents because of its rapidity of action. Conversion can occur within a mean of 27 to 33 minutes after start of infusion.39 Like other type III agents, ibutilide has no negative inotropic effects.
Sotalol is a type III antiarrhythmic agent that, despite results of the Sotalol Amiodarone Atrial Fibrillation Efficacy Trial (SAFE-T), has no consistent proven efficacy for restoration of sinus rhythm in either recent onset or persistent AF.2,40 Sotalol use should be reserved for maintenance of sinus rhythm after acute cardioversion with another agent has been successful. It appears to be as effective and better tolerated than quinidine, as effective as propafenone, but less effective than amiodarone for maintenance of sinus rhythm.23 Its use is preferred in patients with coronary artery disease, hypertension without substantial LVH, or those with minimal or no heart disease.2 Sotalol’s beta-adrenergic antagonistic activity is responsible for many of its side effects including fatigue, dizziness, worsening of bronchospasms, hypotension, and bradycardia.
Vaughn Williams class type IC antiarrhythmics
Flecainide prolongs depolarization and can slow conduction in the AV node and the His-Purkinje system, and below, leading to prolongation of the PR interval, increased QRS duration and possibly, first- and second-degree heart block.18,22,39 On the other hand, type IC agents do not have any effect on the QT interval.18,22,39 Flecainide has proven efficacy when initiated within the first 7 days of AF onset (Table 4, page 160).2,17 Generally, type IC antiarrhythmic agents are better than type IA agents because of more favorable adverse events profiles and higher rates of conversion to sinus rhythm.4,41,42 Based on results of the Cardiac Arrhythmia Suppression Trial (CAST), which evaluated post-acute MI patients with asymptomatic, non-life-threatening ventricular arrhythmias, flecainide and other class IC agents should only be used in patients with structurally normal hearts.43 Use in patients with prior MI or abnormal LVEF should be avoided because of negative inotropic effects. These agents can cause heart failure and life-threatening ventricular tachyarrhythmias.17 Aside from cardiac conditions, flecainide may also be limited by its range of noncardiac adverse effects.
Propafenone has a similar mechanism of action to flecainide. Like flecainide, it is most effective when initiated for recent onset AF.2 The Rythmol Atrial Fibrillation Trial, which tested three sustained-released doses of propafenone versus placebo, showed an AF recurrence rate of 69% in the placebo group versus 30%, 42%, and 52% in the propafenone 425-mg, 325-mg, and 225-mg groups, respectively, after 39 weeks of follow-up.2,44 Dose-related adverse effects, including gastrointestinal symptoms were reported. In separate randomized comparison studies of AF maintenance therapy, propafenone proved to be more effective than amiodarone and as effective as disopyramide, but better tolerated.2 Like flecainide, propafenone side effects include hypotension and bradycardia after conversion to sinus rhythm.4 Both agents may also convert AF to slow atrial flutter causing rapid ventricular conduction with a wide QRS complex.4,5 To prevent rapid ventricular rates, a beta-blocker or nondihydropyridine calcium channel blocker should be coadministered to slow AV nodal conduction.4 Propafenone should not be used in patients with heart failure or severe obstructive lung disease due to its beta-blocking activity.
An advantage of type IC antiarrhythmic agents is the option for certain subsets of AF patients to self-administer the medications at home. Benefits of self-administration, or the “pill-in-the-pocket” approach, include decreased hospital or emergency room visits, decreased need for daily antiarrhythmic therapy, and overall decreased cost.41 Caution must be exercised when using this approach depending on AF classification and specific patient characteristics.
Vaughn Williams class type IA antiarrhythmics
Disopyramide increases the action potential duration of normal cardiac cells and decreases the disparity in refractoriness between normal and infarcted myocardium.18 Disopyramide has been inadequately studied for conversion of AF lasting 2 Anticholinergic, peripheral vasoconstrictive, and negative inotropic effects also limit its use.18 Additional research is needed to determine its safety and efficacy for new onset AF.
Procainamide may have a role in the cardioversion of AF if administered within the first 24 hours of onset.2 Procainamide appears less effective than other agents and there is insufficient evidence for its use in persistent AF.2 Moreover, chronic use of this agent is associated with a lupus-like syndrome as well as gastrointestinal disturbances, central nervous system effects, and blood dyscrasias.18 Oral formulations have been discontinued.
Quinidine is one of the most studied antiarrhythmic agents.2 However, its use for new onset AF has fallen out of favor because of its prominent side effects and the availability of more effective agents.2 Torsades de pointes, leading to syncope or cardiac arrest, is of the greatest concern.18,22,39 Diarrhea and thrombocytopenia are also troublesome side effects that often lead to dose reduction or medication withdrawal.12,16 Quinidine is more effective than placebo for maintaining sinus rhythm, but in a meta-analysis of 6 trials, quinidine was associated with a higher incidence of overall mortality.2 Because quinidine can accelerate the ventricular response, use of beta-blockers or nondihydropyridine calcium channel blockers are suggested to slow the AV nodal response.16
Periprocedural anticoagulation
Regardless of the rhythm control strategy used, patients must be started on appropriate periprocedural anticoagulation therapy. The strategy for anticoagulation with rhythm control differs slightly from that when initiated for rate control. The risk for thrombosis increases as the duration of AF extends beyond 48 hours.6 Inpatients who are candidates for urgent DCC can be started on unfractionated heparin prior to cardioversion to decrease thrombosis risk, and appropriate testing must be performed to ensure the absence of thrombosis.6 However, for all other patients, use of a VKA for 3 to 4 weeks prior to conversion to sinus rhythm is indicated.6 All patients require at least 4 to 12 weeks of anticoagulation therapy after sinus rhythm is restored with the goal INR being 2 to 3.6 In patients with permanent AF and accompanying risk factors, life-long antithrombotic therapy is recommended.6
Future management options
Agents that block the renin-angiotensin system (RAS) are currently being considered for prevention of AF development.2 It has been theorized that angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin-II receptor blockers may play a vital role in preventing AF recurrence.2,30,45,46 Several mechanisms have been proposed to explain the effects of RAS blockade on prevention of AF, including decreased atrial stretch, lowered end-diastolic LV pressure and left atrial pressure, prevention of atrial fibrosis, modification of sympathetic tone, alteration in atrial refractoriness, and direct antiarrhythmic effects.30,45,46 Clinical trials have shown that concurrent therapy with ACEIs and antiarrhythmic agents enhances maintenance of sinus rhythm and lowers rates of recurrence after electrical conversion when compared to an antiarrhythmic alone.46 However, the role of these agents in long-term sinus rhythm maintenance requires more clarification before this approach can become a standard of care.
As previously discussed, warfarin is a less-than-ideal medication for long-term treatment of antithrombotic complications secondary to AF, and there is considerable interest in developing new drugs as well as using other currently available medications to improve outcomes. Previous trials of seemingly promising new agents such as ximelagatran, revealed discrepancies between the efficacy and safety profiles.1 Data from the Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE-A) trial, which enrolled patients considered unsuitable for warfarin treatment, suggest a greater reduction in stroke when aspirin is combined with clopidogrel versus using aspirin alone.47 However, like warfarin therapy, combination therapy with clopidogrel and aspirin is associated with an increased risk of major bleeding.47 Dabigatran, a new oral direct thrombin inhibitor, was compared with warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial with a primary outcome of stroke or systemic embolism and a safety outcome of major hemorrhage.48 A 150-mg dose of dabigatran was superior to warfarin (RR, 0.66; 95% CI, 0.53 to 0.82; PREFERENCES
- Lopes RD, Piccini JP, Hylek EM, Granger CB, Alexander JH. Antithrombotic therapy in atrial fibrillation: guidelines translated for the clinician. J Thromb Thrombolysis. 2008;26:167–174.
- Fuster V, Rydén L, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2006;48:854–906.
- Bauman J, Schoen M. Arrhythmias. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, eds. Pharmacotherapy: A Pathophysiologic Approach. 6th ed. New York: McGraw Hill; 2005:321–356.
- Conway EL, Musco S, Kowey PR. Drug therapy for atrial fibrillation. Cardiol Clin. 2009;27:109–123.
- Aronow WS. Etiology, pathophysiology, and treatment of atrial fibrillation: part I. Cardiol Rev. 2008;16:181–188.
- Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133:546S–592S.
- Rogers, S. Management of atrial fibrillation: NICE guideline and its relevance to primary care. Primary Health Care. 2008;18:31–35.
- National Institute for Health and Clinical Excellence (NICE). NICE Clinical Guideline 36. Atrial Fibrillation: The Management of Atrial Fibrillation. June 2006. Available at: http://www.nice.org.uk/guidance/CG36. Accessed April 29, 2010.
- Padanilam BJ, Prystowsky EN. Atrial fibrillation: goals of therapy and management strategies to achieve the goals. Cardio Clin. 2009;27:189–200.
- Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833.
- Van Gelder IC, Hagens VE, Bosker HA, et al; for the Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840.
- Rao S, Julka M, Paruchuri R. Atrial fibrillation: ways to refine your care. J Fam Pract. 2009;58:64–72.
- Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677.
- Crijns H. Rate versus rhythm control in patients with atrial fibrillation. Drugs. 2005;65:1651–1667.
- Blaauw Y, Crijns H. Treatment of atrial fibrillation. Heart. 2008;94:1342–1349.
- Boriani G, Diemberger I, Biffi M, Martgnani C, Branzi A. Pharmacological cardioversion of atrial fibrillation. Drugs. 2004; 64:2741–2762.
- Aronow WS. Treatment of atrial fibrillation and atrial flutter: part II. Cardiol Rev. 2008;16:230–239.
- Micromedex Healthcare Series [Internet database]. Greenwood Village, Colo: Thomson Reuters (Healthcare) Inc. Updated periodically.
- Giardina E, Levy S, Blaustein R. Clinical use of amiodarone. Available at: http://uptodateol.com/patients/content/topic.do?topicKey=~2LsLbmRwYR__EQP&selectedTitle=13~22&source=search_result. Accessed April 30, 2010.
- Wellens HJ, Braguda P, Abdollah H, Dassen HR. A comparison of the electrophysiologic effects of intravenous and oral amiodarone in the same patient. Circulation. 1984;69:120–124.
- Chevalier P, Durand-Dubief A, Burri H, Cucherat M, Kirkorian G, Tonboul P. Amiodarone versus placebo and classic drugs for cardioversion of recent-onset atrial fibrillation: a meta-analysis. J Am Coll Cardiol. 2003;41:255–262.
- Arnsdorf, Knight B, Blaustein R. Antiarrhythmic Drugs To Maintain Sinus Rhythm In Patients With Atrial Fibrillation. Available at: http://www.uptodate.com/patients/content/topic.do?topicKey=~7EGEo6mjPmZZJWE.Accessed April 30, 2010.
- Singh S, Zoble RG, Yellen L, et al. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or flutter. The Symptomatic Atrial Fibrillation Investigative Research on Dofetilide (SAFIRE-D) Study. Circulation. 2000;102:2385–2390.
- Pederson OD, Bagger H, Keller N, Marchant B, Køber L, Torp-Pederson C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: A Danish investigation of arrhythmia and mortality on dofetilide (DIAMOND) substudy. Circulation. 2001;104:292–296.
- Ansani NI. Dofetilide: A new treatment for arrhythmias. P&T. 2001;26:372–378.
- Tsikouris JP, Cox CD. A review of class III antiarrhythmic agents for atrial fibrillation: maintenance of normal sinus rhythm. Pharmacotherapy. 2001;21:1514–1529.
- Banchs JE, Wolbrette DL, Samii SM, et al. Efficacy and safety of dofetilide in patients with atrial fibrillation and atrial flutter. J Interv Card Electrophysiol. 2008; 23:111–115.
- Advisory Committee Meeting of the Cardiovascular and Renal Drugs Division of the US Food and Drug Administration. Multaq®(Dronedarone) Briefing Document. March 18, 2009. Available at: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM134981.pdf. Accessed April 29, 2010.
- Camm J, Savelieva I. New antiarrhythmic drugs for atrial fibrillation: focus on dronedarone and vernakalant. J Interv Card Electrophysiol. 2008;23:7–14.
- Singh B, Aliot E. Newer antiarrhythmic agents for maintaining sinus rhythm in atrial fibrillation. Eur Heart J Supplements. 2007;9:G17–G25.
- Duray GZ, Ehrilkch JR, Hohnloser SH. Dronedarone: A novel antiarrhythmic agent for the treatment of atrial fibrillation. Curr Opin Cardiol. 2010;25:53–58.
- Singh BN, Connolly SJ, Crinjns H, et al; for the EURIDIS and ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med. 2007;357:987–999.
- Hohnloser SH, Crijns H, van Eickels M, et al; for the ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668–678.
- Køber L, Torp-Pederson C, McMurray JJV, et al; for the Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–2687.
- Piccini JP, Hasselblad V, Peterson E, Washam JB, Califf RM, Kong DF. Comparative efficacy of dronedarone and amiodarone for the maintenance of sinus rhythm in patients with atrial fibrillation. J Am Coll Cardiol. 2009;54:1089–1095.
- National Institute for Health and Clinical Excellence (NICE). Dronedarone second draft guidance. Available at: http://www.nice.org.uk/guidance/index.jsp?action=article&o=48099. Accessed on April 20, 2010.
- Singh D, Cingoliani E, Diamond GA, Kaul S. Dronedarone for atrial fibrillation: Have we expanded the antiarrhythmic armentarium? J Am Coll Cardiol. 2010;55:1569–1576.
- Torp-Pederson C, Pederson OD, Køber L. Antiarrhythmic drugs: Safety first. J Am Coll Cardiol. 2010;55:1577–1579.
- Arnsdorf M, Podrid P, Manning W. Restoration of Sinus Rhythm in Atrial Fibrillation: Therapeutic Options. Available at: http://www.uptodate.com/patients/content/topic.do?topicKey=~6ttXNuD_qDnnbKN. Accessed May 3, 2010.
- Singh BN, Singh SN, Reda DJ, et al; for the Sotalol Amiodarone Atrial Fibrillation Efficacy Trial (SAFE-T) Investigators. Amiodarone versus sotalol for atrial fibrillation. N Eng J Med. 2005;352:1861–1872.
- Lesher B. “Pill-in-the Pocket” approach to treating atrial fibrillation. Pharmacist’s Letter/Prescriber’s Letter. 2005;21(210108):1–4.
- Khan IA. Pharmacological cardioversion of recent onset atrial fibrillation. Eur Heart J. 2004; 25:1274–1276.
- The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary Report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–412.
- Pritchett EL, Page RL, Carlson M, Undesser K, Fava G. Efficacy and safety of sustained-release propafenone (Propafenone SR) for patients with atrial fibrillation. Am J Cardio. 2003;92:941–946.
- Schmieder RE, Kjeldsen SE, Julius S, McInnes GT, Zanchetti A, Hua TA. Reduced incidence of new-onset atrial fibrillation with angiotensin II receptor blockade: the VALUE trial. J Hypertension. 2008;26:403–411.
- Novo G, Guttilla D, Fazio G, Cooper D, Novo S. The role of renin-angiotensin system in atrial fibrillation and the therapeutic effects of ACE-Is and ARBs. Br J Clini Pharmaco. 2008; 66:345–351.
- Connolly SJ, Pogue J, Hart RG, Hohnloser SH, Pfeffer M, Yusuf S. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360:2066–2078.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; and the RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139–1151.
FDA Approves Pfizer’s Gene Therapy for Beqvez for Hemophilia
April 26th 2024Beqvez (fidanacogene elaparvovec) is priced at $3.5 million, which is on parity with Hemgenix, the first one-time therapy to treat adults with hemophilia B. Pfizer’s warranty will refund insurers and continue to provide coverage for patients if they change insurers.
