- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Hospira Recalls One Lot of Propofol
Particulates were seen in a single vial, which could lead to blockage of blood vessels, including decreased blood flow to the brain, heart attack, and pulmonary embolus.
Hospira, a Pfizer company, is voluntarily recalling one lot of propofol injectable emulsion, (containing benzyl alcohol) because of a visible particulate seen in a single vial. Propofol is an intravenous general anesthetic and sedation drug for use in the induction and maintenance of anesthesia or sedation.
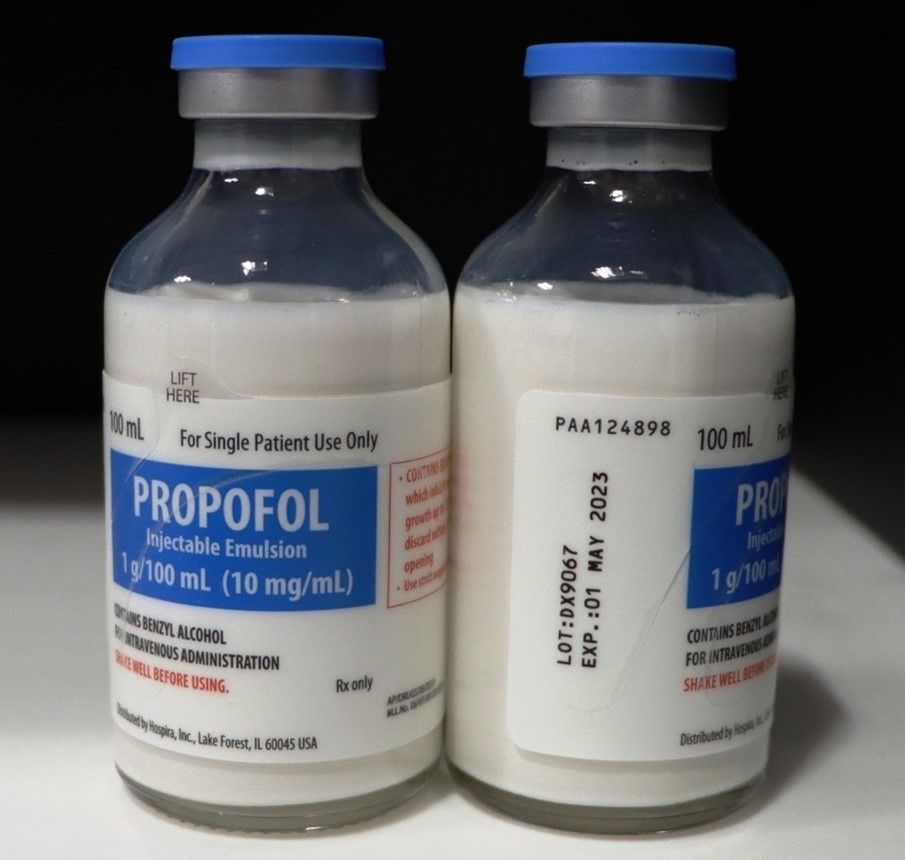
The effected lot is DX9067, with an expiration of May 1, 2023.
Patients receiving the impacted product are at risk of experiencing life-threatening adverse clinical effects including but not limited to: blockage of blood vessels, including decreased blood flow to the brain, heart attack, pulmonary embolus, and tissue necrosis. Hypersensitivity reactions and transmission of infectious disease can also occur.
Hospira has not received reports of any adverse events.
