- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
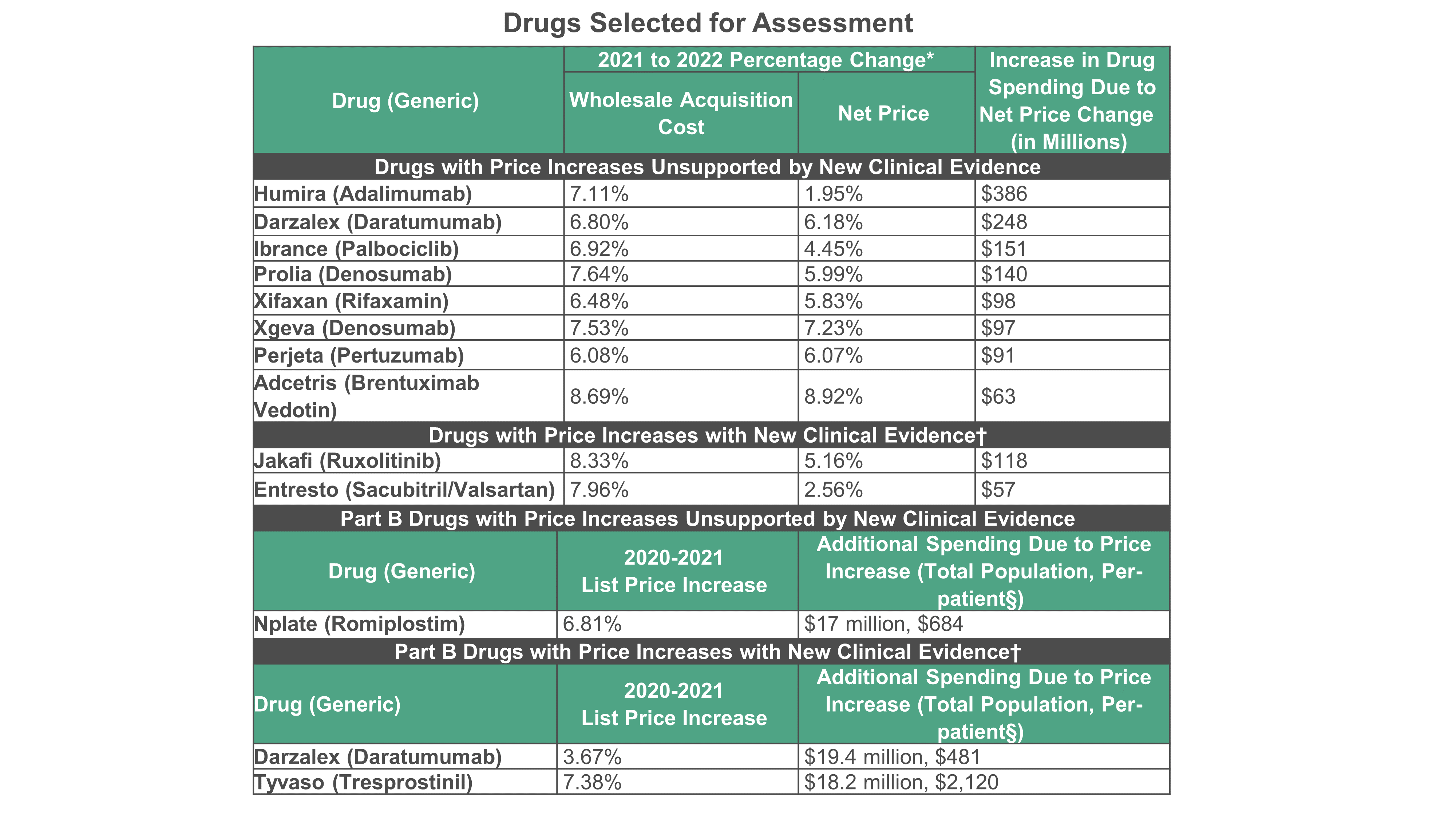
ICER: Humira Among Drugs that had Unsupported Price Increases in 2022
Eight of 10 drugs reviewed by Institute for Clinical and Economic Review were not supported by clinical evidence.
Among 10 drugs with high 2022 price increases, eight were not supported by new clinical evidence, according to a new report by the Institute for Clinical and Economic Review (ICER). This, ICER executives said, accounted for an additional $1.27 billion in costs over one year. (See table below for full list.)
Additionally, one of three Medicare Part B drugs with high list price increases in 2021 lacked adequate supporting new evidence, directly raising annual out-of-pocket expenses for Medicare patients by up to $680 per year, ICER said.
David Rind, M.D.

“We continue to see list price increases above inflation for many of the most costly drugs,” said David Rind, M.D., ICER’s chief medical officer, said in a press release. “List price increases can present real hardships to patients who must pay deductibles or coinsurance.”
For this analysis, ICER researchers used data from SSR Health to determine the 250 drugs with the largest sales revenue in 2022. They excluded drugs that had increases in wholesale acquisition costs that were less than 2% greater than the medical consumer price index. The remaining drugs were assessed to whether increases in spending were from increases in price or volume.
ICER researchers then generated a list of the top 10 drugs whose price increases led to an increase in spending, as well as an additional three drugs with the highest spending by the Centers for Medicare and Medicaid Services. Researchers then assessed new clinical evidence to determine if that would less to improvement in net health benefit.
ER reviewed the quality of any new evidence using the evidence grading system called GRADE, which classifies the evidence into high, moderate, low or very low, based on the quality of the evidence, risk/benefit balance, preferences of the patients and physicians, and costs. ICER notes in its report that it did not perform a cost-effectiveness analysis; this analysis looks at whether new evidence existed that could justify a price increase.
Topping the ICER’s list of unsupported prices is AbbVie’s arthritis drug Humira (adalimumab). The report found that over full year 2022, the wholesale acquisition cost increased 7.11% and the next price increase was 1.95%, which ICER said led to $386 million in additional spending. ICER’s analysis of the literature and of AbbVie’s submitted data met ICER’s criteria of new moderate- to high-quality evidence on the benefits
One drug, Janssen’s multiple myeloma therapy Darzalex (daratumumab), appears twice: as one of eight drugs on the main list as having a price increase unsupported by new clinical evidence and also as one of two drugs on the separate Medicare list judged as having a price increase with new clinical evidence. ICER researchers noted that the time period for the Medicare analysis was different, from 2020 to 2021 because this was the most recent available data from CMS.
In the main analysis, the price of Darzalex in 2022 increased by about 6.8%, while its estimated net price increased by 6.18%, which led to estimated increase in drug spending of $248 million. None of the clinical studies that ICER reviewed met their criteria for moderate- to high-quality evidence on the benefits. In the Medicare analysis of pricing information for 2021 found that the change in spending per unit of Darzalex increased by about 3.67%. One study ICER assessed met their criteria for moderate- to high-quality evidence on the benefits.
Additionally, Amgen’s denosumab appears with two brand names, Prolia and Xgeva. These products have different indications — Prolia treats patients with osteoporosis and Xgeva prevents bone-related issues in patients with multiple myeloma and patients with bone metastases. They also have different dosing regimens and pricing.
Over 2022, the wholesale acquisition cost of Prolia increased about 7.64%. The net price increased by about 5.99%, which resulted in an increase in drug spending of $140 million. Xgeva’s wholesale acquisition cost increased about 7.53%, while its estimated net price increased by 7.23%, which resulted in estimated increase in drug spending of $97 million. ICER’s review of the clinical evidence for both drugs did not meet their criteria for evidence of improvement in benefits.
Two drugs in the main list met ICER’s criteria for additional evidence to support a price increase: Incyte’s Jakafi (ruxolitinib) and Novartis’ Entresto (sacubitril/valsartan). Jakafi treats patients with myelofibrosis and the blood cancer polycythemia vera. In 2022, the wholesale acquisition cost of Jakafi increased about 8.33%, while its estimated net price increased by 5.16%, which resulted in an estimated increase in drug spending of $118 million. ICER researchers found that two trials — MAJIC-PV and REACH 3 — provided new data that qualified as moderate- to high-quality.
Entresto, which is approved to reduce the risk of cardiovascular death and hospitalization and to treat heart failure, had an increase in the wholesale acquisition cost of 7.96%, while its estimated net price increased by 2.56%. This led to an increase in spending by $57 million. New evidence from the PERSPECTIVE trial found that Entresto had a positive impact on cognitive function in patients with heart failure. ICER rated this trial as providing moderate quality because of the possibility of bias resulting from 28% being lost to follow up and the trial has not been peer reviewed.
Source: Institute for Clinical and Economic Review

Payers Recognize the Benefits, but Still See Weight Loss Drugs through a Cost Lens
April 12th 2024Jeffrey Casberg, M.S., R.Ph., a senior vice president of clinical pharmacy at IPD Analytics LLC, a drug intelligence firm that advises payers and pharmaceutical companies, talks about how payers are thinking about weight-loss drugs.
Humira Biosimilars Have a Slow Uptake, Finds Samsung Bioepis Report
April 8th 2024Caps on Medicare Part D cost sharing as a result of the Inflation Reduction Act, could reduce members’ financial incentive for switching to a biosimilar, suggests the newest Samsung Bioepis Quarterly Biosimilar Market Report.
