- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
ICER Review Leads to Formulary Changes
While ICER found that overall major payer coverage policies are in line with the organization’s fair access criteria, many payers do not provide adequate transparency into clinical coverage criteria.
Greater transparency into payer coverage policies can lead to positive change, finds the second Barriers to Fair Access report by the Institute for Clinical and Economic Review (ICER). During ICER’s assessment, five payers revised policies for 11 drugs in ways that bring coverage in line with the research institute’s fair access criteria. Most of the changes made reflect tier placement, but some reflected clinical eligibility criteria.
One example of these changes is Oxbryta (voxelotor) for sickle cell disease in which Anthem updated clinical eligibility criteria to include patients four years and older. In December 2021, the FDA granted an accelerated approval to Global Blood Therapeutics's Oxbryta for pediatric patients aged four up to 11 years. The agency had previously granted accelerated approval for Oxbryta for patients aged 12 years. Global Blood Therapeutics is now part of Pfizer.
ICER’s fair access analysis found that overall major payer coverage policies for the 19 drugs that were reviewed demonstrated high concordance with many fair access criteria related to cost sharing, clinical eligibility, step therapy, and provider restrictions. But ICER also found that many payers do not provide adequate transparency into clinical coverage criteria, and prior authorization forms can have dozens of questions, which puts additional burdens on physicians and their staff.
Sarah K. Emond

“Payers, pharmacy benefit managers, and drug makers all have a role to plan in ensuring fair pricing and fair access to prescription drugs; while much attention is paid to the prices chosen by drug makers, this second annual Barriers to Fair Access report shows that the payer community has a critical role to play in ensuring access,” Sarah K. Emond, MPP, executive vice president and chief operating officer at ICER, said in a press release. “While many of the concordance levels for adhering to fair access criteria were high, we know that patients still face significant barriers to access because of policy choices made by payers. Because our annual exercise, which shines a light a small number of payer policies, leads to payers improving the fairness of their coverage policies, we are demonstrating that transparency and analysis can help the system move to what we all want – fair prices and fair access.”
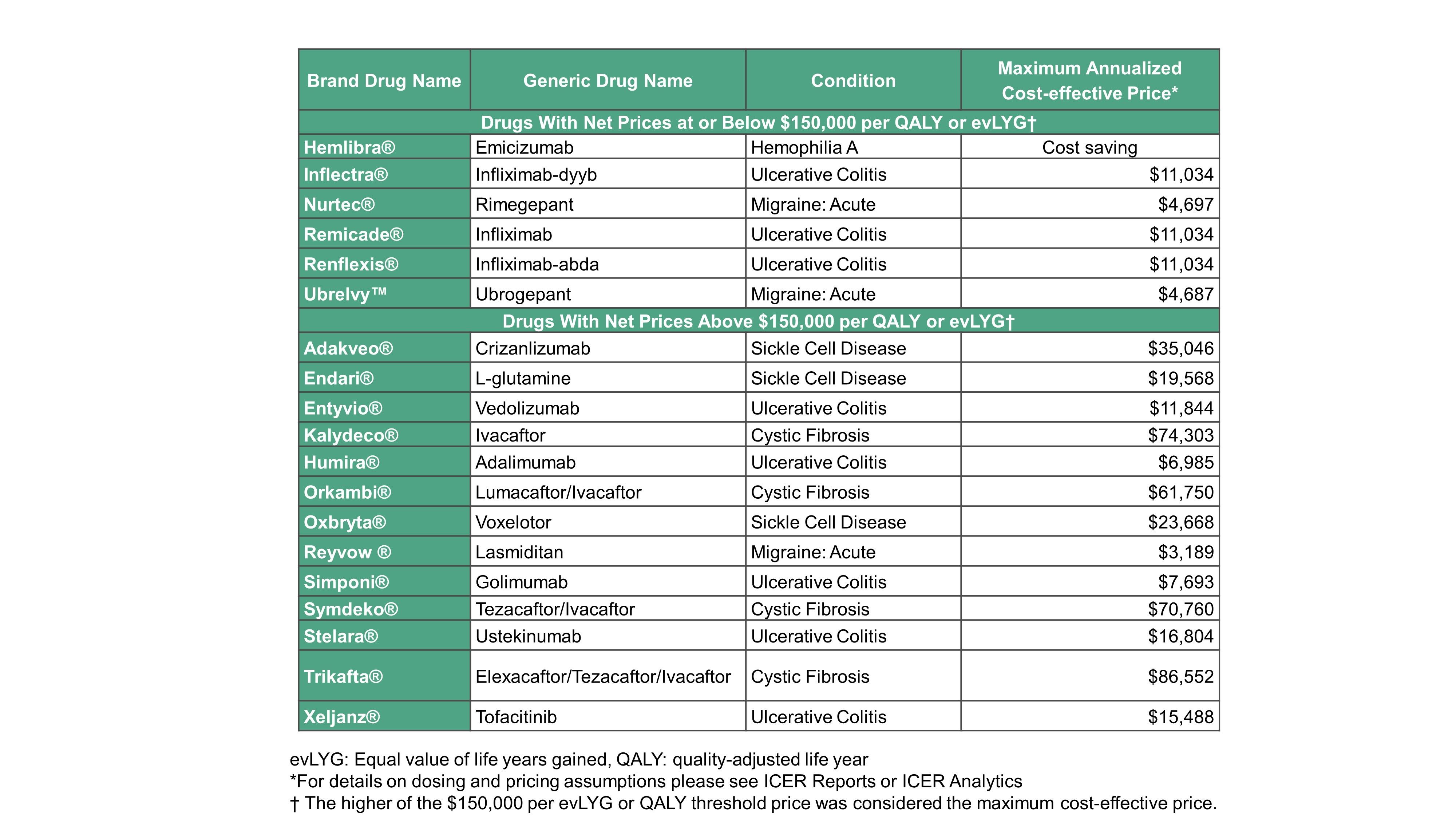
ICER used formulary coverage information from MMIT Analytics Market Access Database to evaluate the insurance coverage policies of the largest formularies of leading payers against ICER’s fair access criteria for the 19 drugs reviewed by ICER in 2020. (See Table below for the list of drugs included in this review.) Payer formularies assessed included the 15 largest commercial formularies in the United States; the Veterans Health Administration (VHA); and formularies of the two largest state ACA exchange health plans. Together, these formularies represent coverage policies governing pharmaceutical access for about 55 million Americans.
ICER reviewers found a high level of alignment between coverage policies and fair access criteria across the formularies with the highest number of covered lives of large private payers and the VHA in the United States. Across all relevant payer policies, ICER gave concordance ratings of 70% for cost-sharing policies of drugs that ICER found to be reasonably priced, 96% for clinical eligibility criteria, 98% for step therapy criteria and 100% for prescriber restrictions.
There was a high rate of concordance overall with ICER’s fair access criteria. Genentech’s hemophilia drug Hemlibra (emicizumab) was the only drug for which more than a single formulary did not meet fair access criteria. ICER found that four payers — Elixir, Cigna, MedImpact and Florida Blue HIX — in their pharmacy benefit coverage of this drug have restrictions based on severity of disease defined by having a history of bleeding events, which is not in the label nor supported by clinical guidelines.
Several drugs were not in concordance with ICER’s step therapy criteria for fair access. For example, for Eli Lilly’s migraine therapy Reyvow (lasmiditan), three payers — United Healthcare, OptumRx, and BCBS MI — require patients to step through two generic triptans and both Ubrelvy and Nurtec (four steps total) before accessing Reyvow. This exceeds ICER’s fair access criteria of a three-step limit.
ICER also conducted two exploratory analyses — for Pfizer’s Nurtec (rimegepant) and AbbVie’s Ubrelvy (ubrogepant) for acute migraine and AbbVie’s Humira (adalimumab) and Pfizer’s Xeljanz (tofacitinib) for ulcerative colitis. Reviewers assessed whether payers provide sufficient transparency into clinical eligibility criteria and cost sharing requirements, as well as an analysis of the burden created by the number of prior authorization questions that providers must complete.
In the exploratory transparency analysis, 16 of 18 (89%) of payers made tiering information available. Adequate information on clinical coverage criteria were provided by 9 of the 13 (64% health insurers and 1 of the 5 (20%) pharmacy benefit managers. In the exploratory analysis of prior authorization documentation burden, the forms requiring provider input had median numbers of questions that ranged from 25 to 36 across the set of migraine and ulcerative colitis drugs, with the full range of the number of questions across payer forms extending from 22 to 71.
Related: Shining Light on the Barriers Payers Place on Medications
In May 2022, ICER released its protocol for this review. The organization had outlined a set of criteria for establishing for how it would assess fair access in a white paper in September 2020. The company developed four key areas for reviewing fair access: cost-sharing, eligibility criteria, step therapy, and prescriber restrictions. These areas aim to balance the need for cost control with appropriate cost-sharing and utilization management strategies.
Source: Institute for Clinical and Economic Review
Payers Recognize the Benefits, but Still See Weight Loss Drugs through a Cost Lens
April 12th 2024Jeffrey Casberg, M.S., R.Ph., a senior vice president of clinical pharmacy at IPD Analytics LLC, a drug intelligence firm that advises payers and pharmaceutical companies, talks about how payers are thinking about weight-loss drugs.
Humira Biosimilars Have a Slow Uptake, Finds Samsung Bioepis Report
April 8th 2024Caps on Medicare Part D cost sharing as a result of the Inflation Reduction Act, could reduce members’ financial incentive for switching to a biosimilar, suggests the newest Samsung Bioepis Quarterly Biosimilar Market Report.
