- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
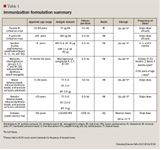
Immunization update: A 2011 retrospective on product changes and new recommendations
Vaccines are one of the greatest achievements of biomedical science and public health. Proper vaccination plays a critical role in the reduction of vaccine-preventable disease and related morbidity and mortality.

Key Points
Abstract
Vaccines are one of the greatest achievements of biomedical science and public health. Proper vaccination plays a critical role in the reduction of vaccine-preventable disease and related morbidity and mortality. Despite levels of vaccine-preventable diseases being at or near record lows, many still persist due to numerous underimmunized children, adolescents, and adults. In addition to morbidity and premature deaths, vaccine-preventable diseases impose a high societal and economic burden, including decreased school and work productivity, increased doctor's visits, and hospitalizations. The Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention releases updated immunization schedules annually. In the 12 months that span schedule updates, new evidence becomes available and product changes occur. New products are approved by FDA and existing products may obtain approval for additional formulations or further indications. This article reviews certain recommendations and product changes that have occurred during 2011 since publication of the immunization schedules in January/February 2011. The covered vaccines include influenza intradermal and high-dose, herpes zoster, human papillomavirus, quadrivalent meningococcal conjugate, and tetanus toxoid. ACIP guidance affects vaccine practices; pharmacists' knowledge of the most current recommendations can enhance patient education, improve patient care, and potentially result in increased vaccination rates. (Formulary. 2012;47:58–74.)

Immunity involves the body's ability to discriminate "self" from "non-self," allowing the immune system to attack foreign antigens and pathogens in order to protect us from disease, while sparing our own tissues.5,7 Immunity is typically very specific to a particular organism or group of similar organisms and is generally noted by the presence of antibodies.6 Immunity can be acquired by 1 of 2 methods, passive or active. Both can be obtained by either natural or artificial means. Passive immunity is acquired by the transfer of preformed antibodies to unimmunized individuals. This method can occur naturally, as in the transplacental passage of maternal antibodies to a fetus. Artificial passive immunity is achieved by the administration of human immune gamma globulin or antitoxin. Due to lack of memory, this passive immunity will dissipate if not accompanied by active production of antibodies. Active immunity requires exposing an unimmunized person to a pathogenic agent either naturally by exposure to the disease or artificially through vaccination. The largest benefit of vaccination is avoidance of the disease and related complications. Whether obtained naturally or artificially, active immunity confers the additional benefit of immunologic memory, which persists for years and may be permanent. This memory allows B cells to replicate and reestablish protection upon future exposure to the same pathogen.6–8
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
