- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
The impact of angiotensin II receptor blocker potency on the clinical outcomes of stroke, acute myocardial infarction, or death
Angiotensin II receptor blockers (ARBs) have been demonstrated to reduce morbidity and/or mortality in patients with chronic heart failure (CHF), acute myocardial infarction (AMI), type 2 diabetes, and hypertension. Although as a class ARBs share a common mechanism of action, potency among the agents varies. Higher-potency ARBs (candesartan, irbesartan, olmesartan, and telmisartan) may demonstrate improved 24-hour blood pressure control, suggesting that these agents may have superior clinical event reduction potential versus lower-potency agents (eprosartan, losartan, and valsartan). We conducted a meta-analysis of randomized, controlled trials that evaluated the effect of ARBs on clinical outcomes. A systematic literature search of MEDLINE from 1966 through December 2006 was conducted using specific search terms. Studies that met the following criteria were included: randomized; not angiotensin-converting enzyme (ACE) inhibitor-controlled; incorporation of monotherapy with ARBs in 1 or more of the treatment..

Key Points
Abstract

Large clinical trials have demonstrated that angiotensin II (AT II) receptor blockers (ARBs) have numerous beneficial clinical effects, including reducing morbidity and mortality in patients with chronic heart failure (CHF), providing efficacy similar to that of angiotensin-converting enzyme (ACE) inhibitors in high-risk acute myocardial infarction (AMI) patients, reducing the rate of diabetic nephropathy progression in patients with type 2 diabetes, and improving clinical outcomes in patients with hypertension.1–12 Because the blockade of AT II produced by ACE or non-ACE pathways decreases aldosterone secretion and antidiuretic hormone release, AT II blockade by ARBs directly or indirectly prevents vasoconstriction, pathogenic cardiac and vascular remodeling, and abnormal renal sodium and free water resorption.13,14

Hypertension is an independent predictor of stroke; the higher the blood pressure, the greater the stroke risk.31,32 Hypertension has also been demonstrated to be an independent risk factor for coronary heart disease, including AMI, and current guidelines recommend tight blood pressure control as part of a primary prevention strategy in patients at increased risk for coronary events.32,33 ARBs have been demonstrated to reduce both cerebrovascular and cardiovascular risk in patients with hypertension.34,35 It is possible that drugs that provide better 24-hour blood pressure control may confer a lower risk of stroke and AMI compared with lower-potency agents.
Although treatment differences between high- and low-potency ARBs are pharmacologically plausible, there are no large, well-designed, head-to-head comparative studies evaluating the differences between these groups on clinical outcomes. Such a trial is unlikely to be conducted, given the large numbers of patients that would be needed, the necessary duration of follow-up, and the projected expense. Meta-analysis is a useful technique when comparative studies are unlikely to be conducted because this technique pools the available information and provides insight regarding the overall effect reflected in the literature. We therefore performed a meta-analysis of large published controlled trials that evaluated ARBs to determine the association between ARB potency and the occurrence of stroke, AMI, and mortality.
MATERIALS AND METHODS
Validity assessment. Jadad scores were calculated to identify studies that used weaker study methodologies.36 This rating scale uses the following quality assessment criteria: description of randomization methods used, description of double-blinding methods used, and description of patient withdrawals and dropouts, as these are inherent controls of bias. One point was given for each satisfied criterion. An aggregate score between 0 and 5 was calculated for each individual trial (0=weakest, 5=strongest); trials scoring <3 were deemed to have lower methodological quality. All studies were reviewed and evaluated by 3 investigators (WLB, CMW, CIC); disagreement was resolved by consensus.
Statistical analysis. Incidence of stroke, AMI, and death were treated as dichotomous variables and were reported as ORs with 95% CIs using a DerSimonian and Laird random-effects model.37 The random-effects model is a standard statistical method that takes into account inherent differences between studies and adjusts for them. This is the preferred method when statistical heterogeneity is present. Statistical heterogeneity was addressed using the Q statistic (P<.1 considered statistically significant). Egger's weighted regression statistics and a visual inspection of funnel plots were used to identify any possible publication bias. The funnel plot is a pictorial representation of each study plotted by its effect size (OR) on the horizontal axis and variance (standard error of the log OR) on the vertical axis. If the plot represents an inverted symmetrical funnel, publication bias is unlikely, but publication bias cannot be excluded when any other configuration is observed. All statistical analysis was performed using StatsDirect statistical software, version 2.4.5 (StatsDirect Ltd, Cheshire, England).
The effect that duration of treatment with ARBs has on the prevention of clinical events such as stroke, AMI, and mortality is unknown. It is possible that longer treatment courses are necessary to see clinical benefit. To reconcile this issue, sensitivity analyses were conducted whereby the meta-analysis was re-analyzed excluding studies with follow-up durations of <1 year. Sensitivity analyses were also conducted to determine the impact of using a fixed-effect model (Mantel-Haenszel fixed-effect model) rather than the random-effects model chosen for the primary analyses.
RESULTS
Study characteristics. Our initial search yielded 6,641 potential citations (Figure 1). Of these, 5,568 citations were excluded by limiting the search to humans; English language; and randomized, controlled trials. Abstracts for 1,073 citations were reviewed; 1,003 were excluded (168 were not randomized, controlled trials; 294 included a renin-angiotensin aldosterone system [RAS] drug comparator; 227 enrolled <100 patients; 126 were duplicate substudies of larger trials; and 189 reported no usable end points). The remaining 70 studies underwent full publication review. Of these, 56 were excluded (14 included an RAS drug comparator, 7 were duplicate substudies of larger trials, and 35 reported no usable end points). Thus, 14 trials (n=46,874 patients) satisfied the inclusion criteria (Table 1).2,7,9–12,38–45 Five trials evaluated low-potency ARBs, and 9 trials evaluated high-potency ARBs. Patient enrollment ranged between 127 and 15,313 patients per study. The included trials evaluated candesartan (n=8), eprosartan (n=1), irbesartan (n=1), losartan (n=2), and valsartan (n=2). Duration of patient follow-up ranged from 12 weeks to 4.8 years.2,7,9–12,38–45
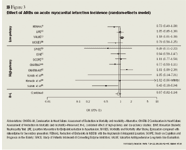
A total of 12 trials (n=41,525) reported AMI incidence.7,9–12,38,39,41–45 ARBs, as a class, had no beneficial effect on AMI incidence versus control (OR=0.97; 95% CI, 0.82–1.14; P=.69) (Figure 3).7,9–12,38,39,41–45 In 8 trials that used high-potency ARBs (n=14,154 patients), no significant difference in AMI incidence was observed between high-potency ARBs and control (OR=0.93; 95% CI, 0.83–1.18; P=.65).7,11,39,41–45 Similarly, in 4 trials that used low-potency ARBs (n=27,371), no significant difference in AMI incidence was observed between low-potency ARBs and control (OR=0.98; 95% CI, 0.80–1.21; P=.88).9,10,12,38 Significant statistical heterogeneity was observed for all ARBs (P=.02), high-potency ARBs (P=.04), and low-potency ARBs (P=.061). The funnel plots looked relatively symmetrical (not shown), but the Egger's weighted regression suggested that publication bias may have been present in the low-potency ARB group (P=.03) but not in the high-potency group (P>.1).
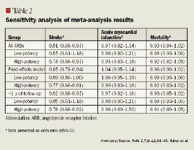
Sensitivity analyses. In the sensitivity analyses, the conclusions from the meta-analysis remained robust to changes. When trials with patient follow-up of <1 year were removed, the overall findings for each analysis remained unchanged.39,44 Similarly, re-analyzing the data using a fixed-effect model did not change the results (Table 2).2,7,9–12,38–45
DISCUSSION
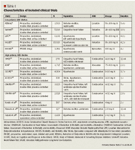
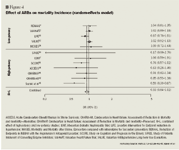
As a class, ARBs reduced the odds of developing stroke (mainly primary) by 19% versus control among the >40,000 patients included in our meta-analysis. The ARB class of drugs did not, however, demonstrate a significant reduction in the incidence of AMI, and the drug class demonstrated only a trend towards reducing mortality. These results are in general agreement with prior meta-analyses.46-48
In our meta-analysis, the use of high-potency ARBs demonstrated a 22% decrease in the incidence of stroke versus control in a population comprising 13,969 patients. Among the 25,858 patients treated with either low-potency ARBs or control who were included in this meta-analysis, low-potency ARBs demonstrated only a nonsignificant 15% reduction in stroke incidence versus control. Although this may suggest that high-potency ARBs provide superior stroke reduction versus low-potency ARBs, we cannot exclude the possibility that the differences observed were due to chance.
The actions of AT II in the brain include blood pressure control, natriuresis, and the release of antidiuretic hormone into the circulation.35 It has been suggested that ARBs provide cerebral protection via an increase in AT II production, which stimulates unopposed AT2 receptors.34 These receptors exert antiproliferative, anti-inflammatory, antifibrotic, and neuronal regenerative properties.49 Thus, the ability of ARBs to block AT1 receptors allows a predominance of AT2-receptor activity in tissues with a normally high AT1:AT2 receptor ratio (such as in the brain). Animal studies have suggested that ARBs exert neuroprotective actions in stroke. This protective effect may be related to the aforementioned upregulation of neuronal AT2 receptors in response to cerebral ischemia in some brain regions.34 Clinical trials have confirmed this benefit. The Losartan Intervention for Endpoint reduction in hypertension (LIFE) study randomized 9,193 patients with hypertension and left ventricular hypertrophy to an atenolol-based or losartan-based regimen.10 Although the agents were associated with similar blood pressure reductions, the losartan-based regimen demonstrated 25% fewer fatal and nonfatal strokes versus atenolol (RR=0.75; 95% CI, 0.63–0.89; P=.001).10 Similarly, the Morbidity and Mortality after Stroke, Eprosartan compared with nitrendipine for Secondary prevention (MOSES) study randomized 1,352 high-risk hypertensive patients who had experienced a cerebrovascular event during the previous 24 months to eprosartan or nitrendipine, a calcium-channel blocker.38 As was observed in LIFE, both agents were associated with similar blood pressure reductions, but a 25% reduction in cerebrovascular events was observed with eprosartan versus nitrendipine.38 Thus, it seems that a portion of the potentially neuroprotective properties of ARBs are independent of blood-pressure lowering.
FDA Approves Combination Therapy for Pulmonary Arterial Hypertension
March 25th 2024J&J’s Opsynvi is single-tablet combination of macitentan, an endothelin receptor antagonist, and tadalafil, a PDE5 inhibitor. It will be priced on parity with Opsumit, which is also a J&J product to treat patients with PAH.
FDA Issues Complete Response Letter for Onpattro in Heart Failure Indication
October 9th 2023Alnylam Pharmaceuticals will no longer pursue this indication of Onpattro and will instead on focus on a label expansion for Amvuttra, which is in phase 3 development to treat patients with cardiomyopathy of ATTR amyloidosis.
