- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Incidence of ADEs in patients readmitted within 30 days of discharge in a tertiary care teaching hospital
Anecdotal reports of hospital readmissions secondary to adverse drug events (ADEs) prompted the pharmacy & therapeutics (P&T) committee at University Hospital, SUNY Upstate Medical University, to authorize a pharmacy-led retrospective review of patient records.

Key Points
Anecdotal reports of hospital readmissions secondary to adverse drug events (ADEs) prompted the pharmacy & therapeutics (P&T) committee at University Hospital, SUNY Upstate Medical University, to authorize a pharmacy-led retrospective review of patient records.
OBJECTIVES
The primary objective of the study was to determine the percentage of patients who were readmitted within 30 days of hospital discharge with an ADE as the primary reason for readmission.
METHODS
Patient readmissions within 30 days of discharge were identified via electronic medical records. The study period ran from January 1, 2005, to June 30, 2005.
For patients to be included in the study, readmission had to occur within 30 days of hospital discharge. If ≥1 readmission occurred within 30 days of hospital discharge, only the first readmission was included in the study.
Charts were reviewed retrospectively for cause of admission, admission and discharge diagnoses, medications before arrival, discharge medications, past medical history, current medical problems, documented signs or symptoms of ADEs, length of hospital stay, days until readmission, and age.
ADEs were identified by a review of notes from the emergency department (ED), as well as admission, discharge, and progress notes; notes were assessed with the Naranjo algorithm for causality. ADEs were considered to be the cause of readmission only if noted as such in the ED admission diagnosis or in the attending physician's discharge diagnosis.
RESULTS
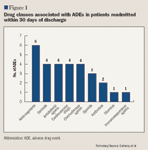
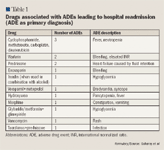
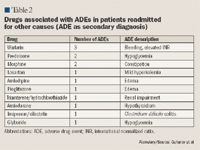
Among reported ADEs, 10.3% were secondary to drug-drug interactions. One patient treated with verapamil and metoprolol experienced bradycardia and syncope. One patient treated with glyburide/ metformin plus glimepiride and 1 patient who used alcohol during insulin treatment experienced hypoglycemia.
LIMITATIONS
This study was limited by a small sample size and a short study period. Additionally, because this study was conducted at a single institution, the results may not be consistent with results from other institutions with different patient populations.
CONCLUSIONS
A high number of hospital readmissions are secondary to ADEs.
Many of these ADEs are preventable as they are caused by lack of monitoring and drug-drug interactions.
Pharmacist discharge counseling and close monitoring of patients on high-risk, narrow therapeutic index drugs are potential solutions for the problem of hospital readmissions associated with ADEs.
Editors' Note:
This article is adapted from a poster originally presented at the 41st American Society of Health-System Pharmacists Midyear Clinical Meeting, Anaheim, CA, December 3–7, 2006.
At the time this manuscript was prepared, Dr Guharoy was director of pharmacy services and associate professor of medicine, University Hospital, SUNY Upstate Medical University, Syracuse, New York. He is now chief pharmacy officer and professor of medicine at the University of Massachusetts Medical Center, Worcester. Dr Bradshaw is a staff clinical pharmacist at St. Joseph's Hospital Health Center, Syracuse, New York. Dr Churmusi, Dr Baxter, Dr Smith, Dr Darko, Dr Probst, and Dr LaHart are clinical pharmacists at University Hospital, SUNY Upstate Medical University.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
