- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Indiplon: A short-acting GABA-A receptor agonist sedative hypnotic for the treatment of insomnia
A number of clinical approaches are utilized in managing insomnia. Indiplon (Pfizer) is a selective non-benzodiazepine sedative hypnotic under consideration by FDA for the treatment of insomnia. Like other agents in its class, indiplon binds selectively to the GABA-A receptors in the brain, promoting sleep.
A number of clinical approaches are utilized in managing insomnia. Indiplon (Pfizer) is a selective non-benzodiazepine sedative hypnotic under consideration by FDA for the treatment of insomnia. Like other agents in its class, indiplon binds selectively to the GABA-A receptors in the brain, promoting sleep. Clinical studies have demonstrated improved efficacy in the treatment of sleep initiation and sleep maintenance with indiplon therapy in patients with acute and chronic insomnia. (Formulary. 2006;41:316–321.)
More than a third of American adults have experienced occasional insomnia or sleep disruption.1 Insomnia is a common disorder characterized by difficulty falling asleep, problems staying asleep, and/or prematurely waking in the presence of adequate opportunity and circumstance for sleep.1–3 Patients may also feel that sleep was poor in quality or non-restorative, although there is controversy as to whether patients with this characteristic share similar pathophysiologic mechanisms with the previously mentioned symptoms. The experience of any of these symptoms may lead to associated daytime functional impairment. It is reported that 30% of the general population have complaints of sleep disruption, with 10% having related adverse daytime consequences.1 Insomnia may be classified as primary idiopathic or the condition may be related to co-morbid conditions. Co-morbid insomnia includes conditions resulting in chronic somatic or physical complaints or pain that may disrupt sleep, gastrointestinal disorders, cardiopulmonary disorders, and psychiatric diseases, particularly depression and substance abuse disorders.1–3 Associated sleep disorders such as obstructive sleep apnea, restless legs syndrome, or periodic limb movement disorder can also contribute to insomnia. Insomnia may be further classified as acute or chronic. Traditionally a 6-month duration of the above symptoms has been used to define chronic insomnia; however, a recent National Institutes of Health consensus statement on insomnia defined chronic insomnia as 30 days or more of the previously mentioned symptoms.1 Chronic insomnia affects 1 in 10 American adults, has a negative impact on quality of life, and can be costly to individuals, society, and the healthcare system.
Pharmacologic treatments for insomnia include the use of hypnotics that act at the benzodiazepine GABA-A receptor, which include benzodiazepines (eg, estazolam, flurazepam, quazepam, temazepam, and triazolam) and non-benzodiazepine agents (eg, zolpidem, zaleplon, and eszopiclone). Indiplon (Pfizer) is one such non-benzodiazepine agent in late-stage development that binds selectively to the GABA-A receptors in the brain. Ramelteon, the newest FDA approved hypnotic for the treatment of insomnia, exerts an agonist effect on melatonin receptors in the brain, inducing sedation.4
In addition, many medications not primarily indicated for insomnia have been used for the management of insomnia. These medications include antidepressants, antihistamines, antipsychotics, barbiturates, and herbal agents (melatonin, valerian, L-tryptophan). Although insomnia is often a chronic condition, most of the medications used for treatment are only indicated for short-term use, 35 days or less.5–7 Patients may also use alcohol for its sedative properties to overcome insomnia. In fact, according to the 2005 National Sleep Foundation "Sleep in America" poll, the most commonly used sleep aid in America is alcohol.8
Chemistry and pharmacology
Indiplon is a short-acting pyrazolopyrimidine sedative hypnotic that binds selectively to the GABA-A receptors in the brain, thereby promoting sleep.9 In vitro experiments conducted in rat brain membranes demonstrated reversible binding and high GABA-A receptor affinity. GABA shift experiments and patch-clamp recordings with cultured rat cortical neurons confirmed indiplon's full agonist efficacy through GABA-A potentiation. Like other non-benzodiazepine hypnotic agents, indiplon has been shown to selectively bind to the Alpha1 subtype of the GABA-A receptor. The selectivity for this subunit in comparison to the Alpha2, Alpha3, or Alpha5 subunits is estimated to be a factor of 10. It is proposed that this receptor's selectivity will minimize adverse reactions associated with benzodiazepine use.10
PHARMACOKINETICS
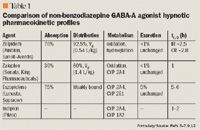
Indiplon is metabolized to 2 major metabolites. N-desmethyl-indiplon is formed by the cytochrome P450 (CYP)3A4 and CYP1A2 metabolic pathways. The other metabolite, N-deacetylindiplon, is formed by microsomal carboxylesterases.9 The pharmacologic activity of these metabolites has not been reported. Results from pharmacokinetic studies suggest little risk of hepatic isoenzyme inhibition or induction by indiplon.9
A study of 30 healthy young male subjects (age range 22–41 y) following administration of either placebo, or indiplon 10, 30, or 45 mg for 14 consecutive days failed to demonstrate the presence of pharmacokinetic tolerance following repeat doses.14
Clinical trials

In a similar study, 593 healthy adults received indiplon IR 10 or 20 mg, or placebo.16 Patients from both of the indiplon dosing groups experienced better subjective and objective sleep onset and total sleep time, as well as sleep efficiency and quality.
The effectiveness of indiplon IR was demonstrated in a 35-day study with 194 chronic insomnia patients (Diagnostic and Statistical Manual [DSM]-IV primary insomnia criteria with ≥3 months duration) who were treated with indiplon 10 or 20 mg or placebo.17 In addition to subjective measurements, polysomnographic recordings were conducted at the beginning, middle, and end of the treatment period, and during the first 2 single blind discontinuation nights. These recordings determined latency to persistent sleep was significantly better for both of the indiplon doses at all time points compared with placebo. After abrupt single-blind discontinuation, there was no evidence of rebound or withdrawal.
The use of indiplon IR has also been examined in elderly patients. In one outpatient study of 358 elderly patients (aged 65–80 y) with primary insomnia, subjects were administered indiplon IR 5 or 10 mg, or placebo for 2 weeks.18 Compared with placebo, both of the indiplon IR doses significantly reduced the reported time to sleep onset during both weeks (P<.0001). Additionally, the number of awakenings, reported total sleep time, and sleep quality were significantly improved during the 2 weeks (all P<.0001). Patients in the 10 mg group also experienced a reduction in wake after sleep onset (WASO). A low percentage of patients discontinued treatment due to adverse effects.
In another study, 4-period crossover, 42 elderly patients (age range 65–82 y) with chronic insomnia were administered indiplon IR 5, 10, or 20 mg, or placebo.19 The indiplon treatment groups demonstrated significantly better objective and subjective sleep onset times (P<.001). Patients administered 10 or 20 mg experienced longer total sleep times (P=.027 and P<.001, respectively).
Treatment of middle of the night awakenings with indiplon IR was examined in a 4 week subjective study of 260 adults with chronic insomnia characterized by prolonged night-time awakenings and difficulty returning to sleep.20 Subjects were randomized to indiplon IR 10, 20 mg, or placebo and instructed to use treatment as needed for nighttime awakening if ≥4 hours remained available for sleep. Both of the indiplon IR doses were associated with significantly shorter time to sleep onset as well as an improvement in average reported post-dosing total sleep time compared with placebo at all of the measured time points. Indiplon IR use was not associated with next-day sleepiness.

Modified-release (MR) formulation. The efficacy of indiplon MR was analyzed in a single night pharmacokinetics study of 36 healthy males (aged 19–42 y).22 Volunteers were subjected to repeated venipunctures for plasma drug concentrations throughout the study night. The venipuncture model effectively disrupted normal sleep patterns and therefore induced transient insomnia. Subjects received placebo or indiplon MR 40 mg at 10 pm. Sleep onset values, total sleep duration, and sleep quality were significantly better for the indiplon group compared with placebo (P<.001, P=.02, and P=.04, respectively).
A 4-week crossover study was conducted in 211 outpatient adult subjects to determine the efficacy of indiplon MR in the treatment of chronic insomnia (≥3 months duration).23 Subjects received placebo for 2 weeks, and then either indiplon MR 30 mg or placebo every night for 2 weeks. During both of the treatment weeks, the subjects in the indiplon MR treatment group reported significantly better total sleep time, WASO, number of awakenings, time to sleep onset, and sleep quality compared with subjects in the placebo group.
The efficacy of indiplon MR for the treatment of chronic insomnia manifested as sleep maintenance problems was demonstrated in a study of 248 adult patients.24 Patients were administered either indiplon MR 15 mg or placebo nightly for 4 weeks. Patients in the indiplon treatment group reported a significant improvement in subjective total sleep time. Patients experienced an average of 1 extra hour of sleep per night.
A sleep laboratory study was conducted to determine the efficacy of indiplon on chronic sleep maintenance insomnia in elderly patients.25 Sixty patients (aged 65–75 y) reporting chronic sleep maintenance insomnia for ≥3 months, received placebo or indiplon MR 10, 20, 30, or 35 mg for 2 consecutive nights. All of the indiplon MR doses demonstrated a significant decrease in the objective time to persistent sleep onset. Also, patients treated with 20, 30, or 35 mg of indiplon MR reported a decrease in the WASO and mean total sleep time (P<.0001).
The efficacy and tolerability of modified-release indiplon was evaluated in a 2-week subjective evaluation in 229 elderly patients (aged 65–85 y) meeting criteria for chronic insomnia.26 Patients were administered indiplon MR 15 mg or placebo. Patients in the indiplon MR treatment group experienced a significant improvement in time to sleep onset, total sleep time, WASO, number of awakenings after sleep onset and total wake time compared with patients in the placebo group. Indiplon MR use was not associated with next-day sleepiness or impairment.

ADVERSE EFFECTS
Safety and tolerability issues have been assessed in multiple indiplon phase 1, 2, and 3 studies. Indiplon has demonstrated minimal potential for adverse effects, tolerance, residual daytime sedation, and respiratory depression. Potential adverse effects and potential next-morning residual sedation or impairment were assessed in clinical efficacy trials with instruments such as the digit symbol substitution test (DSST), symbol copy test (SCT), and visual analogue scale of sleepiness (VAS). Next-morning assessments for bedtime use of indiplon have been similar to placebo with the exception of a modest effect of DSST only at the highest dose, 35 mg, of modified-release indiplon in elderly subjects.25 Thus far, pharmacodynamic or pharmacokinetic tolerance has not been identified with either formulation of indiplon.21,27 Indiplon treatment has not resulted in next-morning residual sedation or impairment.15,20,21,25

DRUG INTERACTIONS
There is little risk of pharmacokinetic interaction between indiplon and coadministered medications secondary to indiplon's weak potential for hepatic isoenzyme inhibition.11 Because indiplon is a substrate for the CYP 3A4 and 1A2 enzymes, there is potential for altered metabolism. However, clinical significance of this effect is unknown. There is a potential for pharmacodynamic interaction with a predictable cumulative sedative effect with indiplon and other CNS depressants. Concomitant administration of indiplon and alcohol to healthy male volunteers resulted in little or no additive pharmacodynamic interaction.8
Dosing and administration
New drug applications have been filed for indiplon IR capsules and MR tablets. Indiplon had not yet received FDA approval at press time. Based on clinical trials, immediate-release indiplon 10 to 30 mg or modified-release indiplon 10 mg to 40 mg once daily at bedtime for 1 month has been effective in patients with chronic insomnia.
Dr Gryskiewicz is a clinical pharmacist at Hartford Hospital in Hartford, Conn. She can be reached at kgryskiewicz@harthosp.org
. Dr Semanco is a clinical pharmacist at Hartford Hospital.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, director of Drug Information Services at Hartford Hospital in Hartford, Conn, and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn; and by Craig I. Coleman, PharmD, assistant professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
Editors' note: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
REFERENCES
1. National Institutes of Health State of the Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults. J Clin Sleep Med. 2005;1:412–421.
2. Schenck CH, Mahowald MW, Sack RL. Assessment and management of insomnia. JAMA. 2003;289:2475–2479.
3. Silber MH. Chronic insomnia. N Engl J Med. 2005; 353:803–810.
4. Rozerem [package insert]. Lincolnshire, Ill: Takeda; 2005.
5. Sonata [package insert]. Philadelphia, Pa: Wyeth; 2004.
6. Lunesta [package insert]. Marlborough, Mass: Sepracor; 2005.
7. Ambien [package insert]. New York, NY: Sanofi-Synthelabo; 2004.
8. Berkowitz DV, Scharf MB, Jochelson P, et al. The coadministration of indiplon (NBI 34060) and alcohol lacks pharmacokinetic and pharmacodynamic interaction. Sleep. 2003;26:A81.
9. Neubauer DN. Indiplon: the development of a new hypnotic. Expert Opin Investig Drugs. 2005;10:1269–1276.
10. Statement on a nonproprietary name adopted by the USAN council. Available at http:// http://www.ama-assn.org/ama/upload/mm/365/indiplon.doc.Accessed/ June 27, 2006.
11. Foster AC, Pelleymounter MA, Cullen MJ, et al. In vivo pharmacological characterization of indiplon, a novel pyrazolopyrimidine sedative hypnotic. J Pharmacol Exp Ther. 2004;311:547–559.
12. Rogowski R, Garber M, Bozigian H, et al. NBI-34060 (a non-benzodiazepine sedative hypnotic): lack of a pharmacokinetic gender effect. Sleep. 2002;25:A415.
13. Jochelson P, Rogowski R, Burke J, et al. The pharmacokinetics of the non-benzodiazepine sedative hypnotic NBI-34060 in elderly subjects. Sleep. 2002;25:A416.
14. Jochelson P, Chen TK, Farber R, Campbell B. Lack of pharmacological and pharmacokinetic tolerance following repeat dosing of indiplon (NBI 34060). Sleep. 2003;26:A85-86.
15. Roth T, Walsh JK, Rogowski R, Farber R, Burke J, Jochelson P. Efficacy and tolerability of indiplon (NBI-34060) solution in healthy adults in a model on transient insomnia. Sleep. 2003;26:A87.
16. Scharf MB, Roth T, Rosenberg R, Lankford DA, Alexander T. Efficacy and tolerability of indiplon IR in the treatment of transient insomnia. Sleep. 2004;27:A267.
17. Walsh JK, Rosenberg R, Roth T, Lankford DA, Jochelson P. Treatment of primary insomnia for five weeks with indiplon-IR. Sleep. 2004;27:A259.
18. Walsh JK, Roth T, Moscovitch A, Farber R, Jochelson P. Efficacy and tolerability of indiplon-IR in elderly patients with primary insomnia. Sleep. 2005;28:A229.
19. Scharf MB, Rosenberg R, Cohn M, et al. Safety and efficacy of immediate release indiplon (NBI-34060) in elderly patients with insomnia. Sleep. 2003;26:A85.
20. Roth T, Zammit G, Scharf MB, Boyd M, Jochelson P. Efficacy and safety of indiplon-IR in adults with chronic insomnia characterized by prolonged nighttime awakenings with difficulty returning to sleep. Sleep. 2005;28:A229.
21. Scharf MB, Black J, Hull S, et al. Long term efficacy and tolerability of indiplon-IR in the treatment of chronic insomnia: results of a double-blind, placebo-controlled 3-month study. Sleep. 2005;28:A229.
22. Jochelson P, Bozigian H, Garber M, et al. The activity of modified release indiplon (NBI-34060) in a transient nighttime venipuncture model. Sleep. 2003;26:A84.
23. Jochelson P, Scharf MB, Roth T, Walsh JK, Garber M. Efficacy of indiplon in inducing and maintaining sleep in patients with chronic sleep maintenance insomnia. Sleep. 2004;27:A262.
24. Neurocrine reports positive phase III efficacy and safety results with indiplon MR tablets in treating adult patients with chronic insomnia [press release]. San Diego: Neurocrine Biosciences; February 16, 2005. Available at: http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-newsArticle&ID=675569&highlight=Accessed/ June 27, 2006.
25. Walsh JK, Lankford DD, Krystal A, et al. Efficacy and tolerability of four doses of indiplon (NBI-34060) modified release in elderly patients with sleep maintenance insomnia. Sleep. 2003;26:A78.
26. Lankford J, Lydiard R, Walsh JK, Seiden D, Jochelson P. Efficacy and tolerability of indiplon-MR in elderly patients with chronic insomnia: results of a double-blind, placebo-controlled, 2 week trial. Sleep. 2005;28:A230.
27. Neurocrine's RESTFUL study demonstrates highly positive long-term efficacy results with indiplon immediate release [press release]. San Diego: Neurocrine Biosciences; March 24, 2004. Available at: http://phx.corporate-ir.net/phoenix.zhtml?c=68817&p=irol-newsArticle&ID=613213&highlight=Accessed/ June 27, 2006.
28. Cohn M, Jochelson P, Gately N, Baron C, Boyd M. Assessment of respiratory effects of indiplon-MR in a CO2 challenge model. Sleep. 2004;27:A261.
29. Hull S, Fogarty C, Vince B, Chediak AD, Jochelson P, Gately N. Safety of indiplon-MR in patients with mild to moderate COPD. Sleep. 2004;27:A261.
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
