- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Interim analysis of RECORD trial provides inconclusive data regarding cardiovascular safety of rosiglitazone
In this multicenter, randomized, open-label trial, it was demonstrated that among patients whose diabetes was not well controlled with either a sulfonylurea or metformin alone, the addition of rosiglitazone (n=2,220) did not increase the risk of cardiac-related hospitalization or death compared with patients who received metformin plus a sulfonylurea (n=2,227).

Key Points
The study by Stephen Nissen, MD, and Kathy Wolski, MPH, that raised concerns about the cardiovascular safety of rosiglitazone is not the only assessment of the drug's safety. The interim results of another study, the Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD) trial, were published in the New England Journal of Medicine (NEJM). The study results were inconclusive.
The primary end point for this trial was hospitalization or death from cardiovascular causes.
A total of 217 patients receiving rosiglitazone as add-on therapy reached the primary end point compared with 202 patients who received a combination of metformin and a sulfonylurea (HR=1.08; 95% CI, 0.89–1.31; P=.43). Patients treated with rosiglitazone did not demonstrate an increased risk of myocardial infarction (MI) (P=.50) or cardiac death (P=.46) compared with those not treated with rosiglitazone.
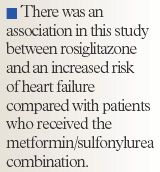
Although the RECORD trial did not demonstrate the same increased risk of MI or cardiac death with the use of rosiglitazone as was demonstrated in the Nissen and Wolski meta-analysis, there was an association in this study between rosiglitazone and an increased risk of heart failure compared with patients who received the metformin/sulfonylurea combination (HR=2.15; 95% CI, 1.30–3.57; P=.003).
The authors stated that the increased risk of heart failure with rosiglitazone use is consistent with results from previous studies of thiazolidinediones. According to the authors: "Although the absolute excess risk was relatively small, this finding is of concern and reinforces advice that patients should be warned of the risk and that thiazolidinediones should not be started or continued in patients with heart failure."
The authors stated that the mean 3.75-year follow-up period did limit the statistical power to identify treatment differences.
In an accompanying editorial, David M. Nathan, MD, director of the Diabetes Center at Massachusetts General Hospital, Boston, and professor of medicine at Harvard Medical School, Boston, agreed with the RECORD trial's authors on this point: "Unfortunately, this interim analysis, performed after a mean of 3.75 years (about 60% of the planned 6-year duration of the study), fails to provide exculpatory evidence."
SOURCES
Home PD, Pocock SJ, Beck-Nielsen H, et al; for the RECORD Study Group. Rosiglitazone evaluated for cardiovascular outcomes-an interim analysis. N Engl J Med. 2007;357:28–38.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471.
Nathan DM. Rosiglitazone and cardiotoxicity-weighing the evidence. N Engl J Med. 2007;357:64-66.
Employers Face Barriers With Adopting Biosimilars
March 1st 2022Despite the promise of savings billions of dollars in the United States, adoption of biosimilars has been slow. A roundtable discussion among employers highlighted some of the barriers, including formulary design and drug pricing and rebates.
