- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
IRA Impact: Lowered Patient Costs, but Also Higher Utilization Management?
Once Medicare’s negotiated price for prescription drugs goes into effect, payers have more of an incentive to steer patients to lower-cost alternatives.
Medicare’s negotiation of prescription drugs could lead to increased access for Medicare patients. This is because of a little talked-about part of the Inflation Reduction Act that requires all Medicare Part D plans to cover each of the selected drugs once the new prices go into effect in 2026. But it could also lead to increased use of payer utilization management efforts to rein in costs.
Two recent reviews look at this part of the law for its impact on patients and physicians. KFF suggests in its new analysis that Medicare’s effort to negotiate drug prices could expand access. In its review, KFF analysts find that currently not all Medicare Part D beneficiaries have coverage for all the 10 drugs that have been selected for the first round of price negotiations.
KFF finds that the share of beneficiaries who have coverage for Fiasp/NovoLog is 60% and 66% have coverage for Stelara, two drugs on the list. Fiasp/NovoLog (insulin aspart) treats diabetes and Stelara (ustekinumab) is treats psoriasis, psoriatic arthritis, Crohn’s and ulcerative colitis. Imbruvica, as a cancer, is considered a protect class, and all plans must coverage.
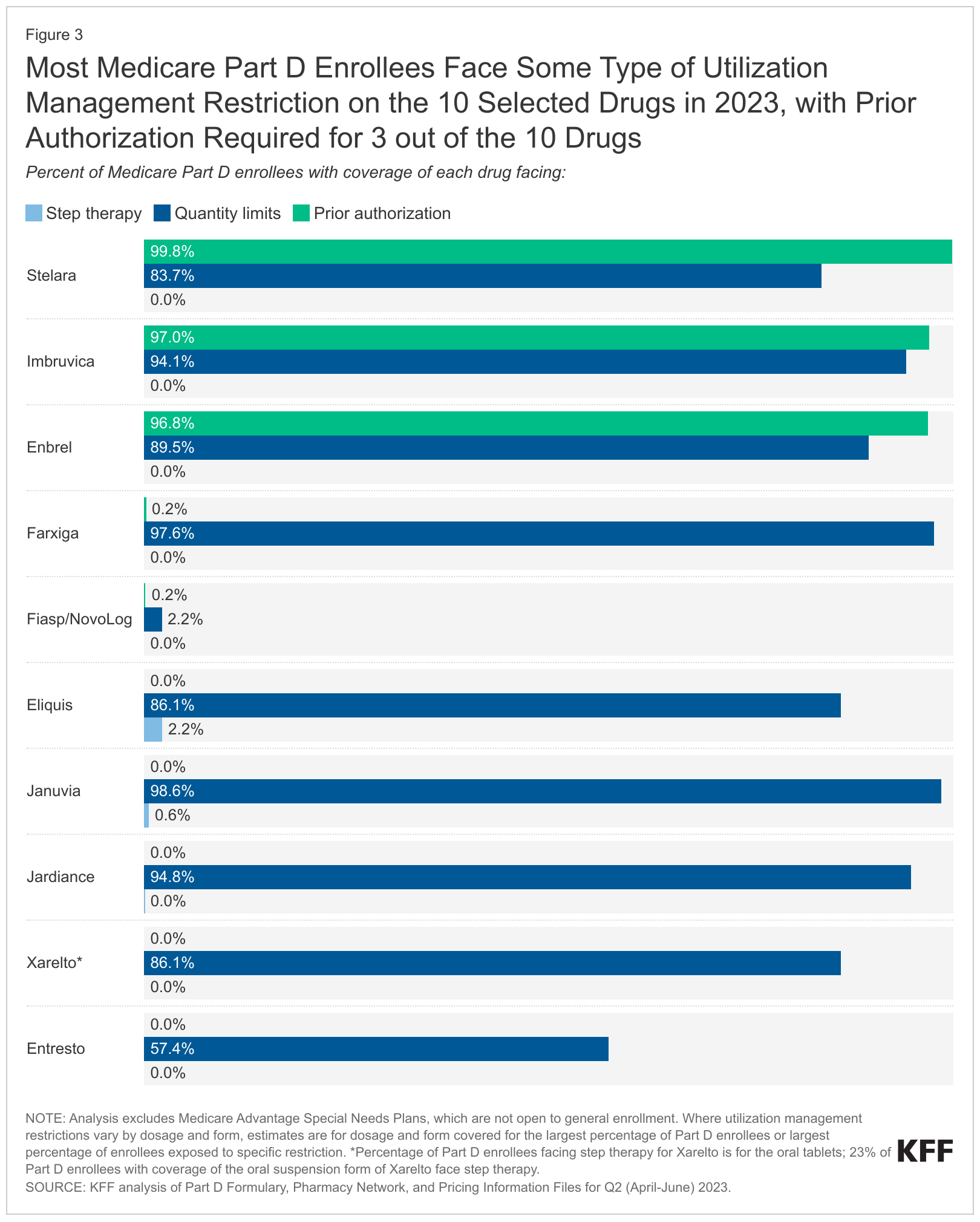
KFF also found that most Part D beneficiaries faced some type of utilization management restriction on the 10 selected drugs. (See Figure below.) Almost all beneficiaries would need to get prior authorization from their plan to begin treatment with antiarthritic drug Enbrel (etanercept), Imbruvica and Stelara. The most common utilization management restriction applied to the 10 selected drugs is quantity limits; step therapy is rarely applied to these drugs.
KFF authors indicated in their brief that CMS intends “to use the annual formulary review process to ensure that all Part D plans cover all dosages and forms of selected drugs during the year that the negotiated prices apply.”
“CMS is concerned that Part D sponsors may be incentivized in certain circumstances to disadvantage selected drugs by placing selected drugs on less favorable tiers compared to non-selected drugs, or by applying utilization management that is not based on medical appropriateness to steer Part D beneficiaries away from selected drugs in favor of non-selected drugs,” Meena Seshamani, M.D., Ph.D., CMS deputy administrator and director of the Center for Medicare, said in a guidance memo released in June.
Even though the IRA also requires plans to justify formulary placement with CMS reviewing use of utilization management tools, industry leaders suggest increased use of prior authorization and step therapy is to be expected.
Mark Campbell, Pharm.D.

Prior authorization the first 10 drugs will likely continue after negotiated prices have gone into effect, Mark Campbell, Pharm.D., RxBenefits’ senior vice president of clinical solutions, said in an interview.
“Utilization, regardless of rebates or net cost, is an important aspect. We need to make sure with expensive therapies that we’re qualifying who is eligible. Now, more than ever, we need to make sure that people are on the right drug, and that it is having the intended effect.”
Analysis from a paper in the Sept. 20, 2023, issue of NEJM Catalyst Innovations in Care Delivery suggests payers may increase their reliance on prior authorization and step therapy requirements as a way to keep costs down.
Authors, led by Amitabh Chandra, Ph.D., professor of Business Administration, Harvard Business School and professor of Public Policy at the Harvard Kennedy School of Government, suggest that with the lower cost sharing that would result from the negotiated prices, payers would have an incentive to use prior authorization and step therapy to steer patients to lower-cost alternatives.
“It remains to be seen whether payers and integrated delivery systems can operationalize utilization management tools in a comprehensive manner by 2025,” the authors write. “However, in the absence of more utilization management, the increase in the use of medicines and an inevitable accompanying increase in premiums would eliminate many of the benefits from lowered cost sharing.”
Source: KFF