- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
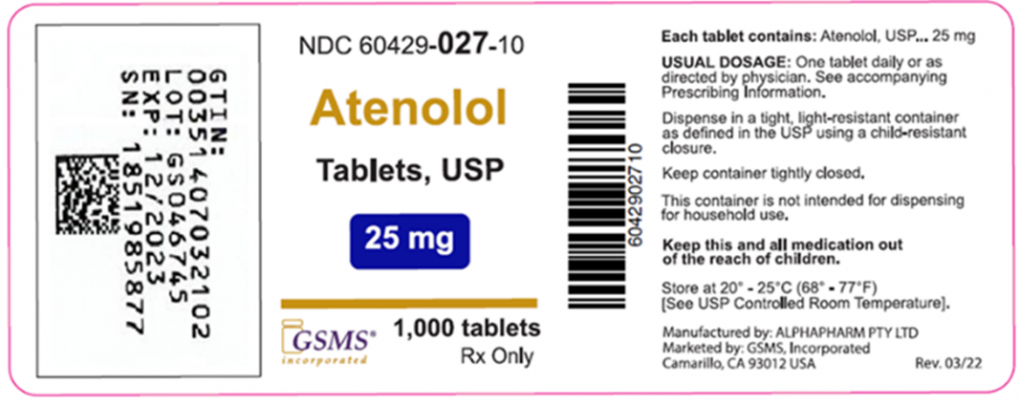
Label Mix Up Leads to Recall of Clopidogrel and Atenolol
In one lot, a bottle containing clopidogrel was mislabeled as atenolol. This lot was sold to AmerisourceBergen and McKesson.
Golden State Medical Supply has initiated a voluntary recall of two products: clopidogrel 75 mg tablets and atenolol 25 mg tablets. The company received a report that a bottle containing Clopidogrel 75mg Tablets produced with lot number GS046745 was mislabeled as Atenolol 25mg Tablets.
This recall only affects products with lot number GS046745. No other clopidogrel or atenolol products marketed by Golden State Medical Supply are impacted. This lot was sold to AmerisourceBergen and McKesson.
Atenolol tablets are indicated to treat patients with hypertension. Clopidogrel is prescribed to lower the risk of having a stroke, blood clot, or serious heart problem for patients who have had heart attack, severe chest pain, or circulation problems.
To date, Golden State Medical Supply has not received any reports of adverse events related to the use of the products as part of this recall.
Patients who suddenly stop taking atenolol are at increased risk for myocardial infarction, hypertensive and arrhythmic adverse events relating to rapid withdrawal of beta antagonism. Patients who are on atenolol are frequently on concomitant anticoagulant and antiplatelet medications and would be at increased risk for bleeding if clopidogrel were added to the regimen