- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Lack of insulin competition high priority for FDA
FDA fast-tracks biosimiliar insulin products to elevate competition.
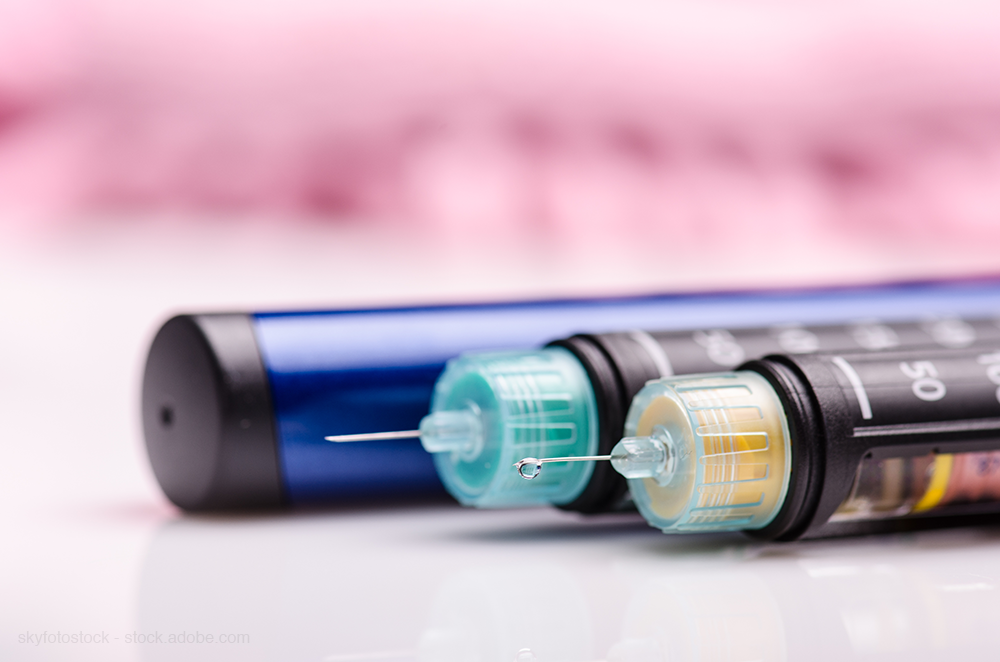
As pharma makers come under fire by Congress for high insulin prices, FDA says it is stepping up efforts to increase competition in the category.
The agency set a public hearing for May 13 to discuss its review and approval of biosimilar and interchangeable insulin products-one of its efforts to lower insulin drug prices.
“Insulin list prices regularly increase by double digits annually. These increases have raised serious concerns about the ability for many patients to access the insulin needed to survive,” said FDA Commissioner Scott Gottlieb, MD, in a statement from the agency. “We understand the urgent need to address the high prices and lack of competition in the insulin market.”
Related: Eli Lilly launches generic insulin ahead of Congressional hearings
One way to lower drug prices is through competition, Gottlieb noted. However, the pathway to approval of the generic and biosimilar versions has been challenging-and some biologic insulin makers have fought competition.
In one exception, Eli Lilly recently introduced a generic version of its insulin lispro injection (Humalog). The list price of the generic Humalog, called Insulin Lispro, will be 50% lower than the branded medication at $137.35 per vial and $265.20 for a 5-pack of KwikPens, Eli Lilly said in a statement.
Eli Lilly, along with other major insulin makers, Novo Nordisk (Tresiba and other brands) and Sanofi (Lantus) were questioned about the price of their drugs at Congressional hearings in March.
One of the factors inhibiting competition is that insulin products are biologics, but historically have been regulated under the FD&C Act rather than the PHS Act, which governs the FDA approval of most biologics.
“Biologics are typically isolated from a variety of natural sources-human, animal or microorganism-and may be produced by biotechnology methods and other cutting-edge technologies. Due in part to the complexities of these products, it has been hard to bring a substitutable generic insulin to the market under the FD&C Act,” Gottlieb said.
Transitioning insulin from the drug to the biologics pathway will open up these products to biosimilar competition. “We’re already seeing robust activity among sponsors seeking to bring forward biosimilar copies of insulin,” Gottlieb said.
Related: Inside Trump’s plan to lower drug prices
The framework for demonstrating that insulin products are interchangeable should also be efficient and achievable. “Bringing forward generic copies of insulin under the old drug pathway was challenging. Generally, it was difficult to prove sameness for a biological product under the Hatch-Waxman pathway for traditional generic drugs. We believe the biosimilar pathway will enable a more robust route for developing lower cost copies of insulin, including products that are fully interchangeable with branded insulins,” Gottlieb said.
As the pathway transition takes place, FDA said it recognizes there are unique challenges associated with bringing biosimilar and interchangeable insulin to market. “We’re working now-in advance of the 2020 transition-to build a solid regulatory foundation for the review and approval of biosimilar and interchangeable insulin products,” Gottlieb said.
To that end, the agency’s public hearing will discuss access to affordable insulin products, as well as the scientific and regulatory issues related to the development and evaluation of biosimilar and interchangeable insulin products.
FDA Issues Complete Response Letter for Pz-Cel to Treat Epidermolysis Bullosa
April 22nd 2024Prademagene zamikeracel is a cell therapy designed to incorporate the functional collagen-producing COL7A1 gene into a patient’s own skin cells. The FDA is asking for additional information on manufacturing practices.
