- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Liposomal bupivacaine: A long-acting local anesthetic for postsurgical analgesia
A look at liposomal bupivacaine, a local anesthetic providing pain relief post-surgery
Abstract
More than 90 million surgical procedures are performed in the United States each year, and providing effective postsurgical pain management is a clinical imperative for every patient undergoing surgery. Infiltration of local anesthetic into the surgical site at closure provides temporary analgesia and is one aspect of the multimodal approach to postsurgical analgesia recommended in current guidelines. The duration of action of bupivacaine HCl and other available local anesthetics is limited, however, lasting 7 hours or less, and patients may experience breakthrough pain before they are able to take or tolerate oral analgesics, thus necessitating the use of strong parenteral analgesics (frequently opioids) in the immediate postsurgical period. A medical need exists to extend the pain relief supplied by bupivacaine HCl, thereby delaying, decreasing, and/or eliminating the potential need for opioids. A new formulation, bupivacaine liposome injectable suspension, is indicated for administration into the surgical site to produce postsurgical analgesia. After local infiltration of liposomal bupivacaine into soft tissue, bupivacaine is released from the multivesicular liposomes over a period of time, resulting in markedly prolonged plasma levels and analgesia. A single dose of liposomal bupivacaine is associated with both pain relief for 72 hours and a 45% reduction in total opioid consumption at 72 hours. (Formulary. 2012; 47:212–226.)
More than 90 million surgical procedures are performed every year in the United States (almost 35 million ambulatory procedures and 56 million inpatient procedures), and, according to several national surveys, approximately 80% of patients undergoing surgery report pain that is moderate, severe, or extreme in intensity during the first 2 weeks postprocedure.1–3 The challenges surrounding postsurgical pain management are complex and multifactorial and require a thoughtful approach. Experts have therefore suggested using a variety of treatment modalities to obtain an optimal outcome after surgery. This "multimodal" or "balanced" approach to analgesia aims to prevent postsurgical pain by using a combination of opioid and nonopioid analgesic drugs that act at different sites within the central and peripheral nervous systems in an effort to reduce pain effectively and minimize opioid use, thus reducing opioid-related side effects.4
Constipation is one of the most common ORAEs because opioids universally decrease gastrointestinal motility.5,7 Not only do they reduce peristalsis in the small and large intestine, they increase the tone of the pyloric sphincter, ileocecal valve, and anal sphincter.5
Nausea or vomiting is also a very common adverse event associated with opioid use.5,7,8 It is especially prevalent in the immediate postsurgical period. A large retrospective analysis (N=434,304) found that 55% of hospitalized patients required treatment for nausea, vomiting, or constipation after analgesic administration and that use of these treatments was almost 5 times more frequent in patients who had received injectable opioids compared with those who had received oral nonopioid analgesics.9
Other common ORAEs include dry mouth, itching/rash, sedation/drowsiness/clouded mental state, dizziness, urinary retention, and myoclonus.2,6,10,11 It has been estimated that 6% to 50% of postsurgical patients develop urinary retention, depending on the location and duration of surgery, the use of opioid therapy, and the need for peri- or postsurgical fluids.12 Most patients with urinary retention require catheterization, which is associated with an increased risk of developing urinary tract infection 12,13 and noninfectious catheter-related complications that can significantly increase the hospital length of stay after surgery.14
Among the more rare events that can occur with opioids, one of the most serious is respiratory depression. All opioids depress the brain's response to carbon dioxide, thereby depressing ventilation. Although this is affected mainly by a reduction in respiratory rate, high doses of opioids can also depress tidal volumes, leading to apnea and even death.5
Opioids are often administered through patient-controlled analgesia (PCA) systems (either epidurally or IV) in the immediate postsurgical period. The set up, maintenance, and monitoring of these systems can be technically challenging, especially in the case of epidural analgesia. Catheter placement can be difficult; the catheter may "migrate," allowing some or the entire dose to leak into the patient's bed, and incorrect positioning may result in paresthesia or insufficient pain control. The most common drawbacks of PCA are the ORAEs and the high frequency of documented medication errors. PCA-related medication errors arise from inadvertent programming mistakes, drug nomenclature errors, battery failures, prescribing mistakes (eg, when converting from the oral to intravenous [IV] form of hydromorphone), patient confusion due to insufficient education, and patient's mistaking the infusion line with the nurse call button, among others.15, 16
Infiltration of local anesthetic into the surgical site at closure provides temporary analgesia and is 1 aspect of the multimodal approach to postsurgical analgesia recommended in current guidelines.5,17 Although this infiltration provides a base platform of postsurgical pain management, the duration of action of conventional bupivacaine HCl and other available local anesthetics is limited, lasting 7 hours or less.18 This short duration of action of bupivacaine has given rise to elastomeric bag technology to extend the local analgesic effect with a pressurized ball containing a local analgesic (ie, bupivacaine HCl) connected to a catheter, which drips the analgesic directly into the wound for an extended period of time, often several days (usually about 3 days). Although this extends the duration of analgesia in the postsurgical site, the Institute for Safe Medication Practices cited the following concerns:19, 20
In October 2011, FDA approved liposomal bupivacaine (bupivacaine liposome injectable suspension) for single-dose infiltration into the surgical site to produce postsurgical analgesia.22 After local administration of liposomal bupivacaine into soft tissue, bupivacaine is released from the multivesicular liposomes over a period of time, resulting in markedly prolonged plasma levels and analgesia for 72 hours with a corresponding reduction in use of opioids. The surgeon infiltrates liposomal bupivacaine before site closure; therefore no tubing or catheters are used and no device programming or nurse training is required.
INDICATIONS
Liposomal bupivacaine is a formulation of bupivacaine HCl, an amide-type local anesthetic/analgesic indicated for single-dose infiltration into the surgical site to produce postsurgical analgesia.23 To date, liposomal bupivacaine has been studied in patients undergoing soft tissue surgery (hemorrhoidectomy, inguinal hernia repair, and augmentation mammoplasty) and orthopedic surgery (bunionectomy and total knee arthroplasty [TKA]). It has not been studied for use in patients younger than 18 years of age.
CHEMISTRY AND PHARMACOLOGY
Liposomal bupivacaine is a new formulation of bupivacaine HCl with a similar pharmacologic profile to the parent drug. The main difference is the new drug delivery system (DepoFoam) formulated to deliver bupivacaine slowly over time to extend its duration of pharmacologic effect. This new formulation is a preservative-free aqueous suspension of multivesicular liposomes containing bupivacaine at a concentration of 13.3 mg/mL (expressed as anhydrous bupivacaine HCl equivalent). Although most of the bupivacaine is encapsulated in the multivesicular liposomes, a small amount (3%) is present as free bupivacaine. This free bupivacaine is released immediately after injection of liposomal bupivacaine into soft tissue. The result is an early effect on local pain receptors, followed by a more prolonged blockade as the rest of the drug is released from the multivesicular liposomes over a period of time. The new drug delivery technology is made up of microscopic, spherical, lipid-based particles arranged as a honeycomb of numerous, nonconcentric, internal aqueous chambers containing the encapsulated drug. Each chamber is separated from adjacent chambers by lipid membranes. In vivo studies have shown that these particles release the encapsulated drug over an extended period of time as the lipid membranes erode and/or reorganize. Release rates are determined by the choice and relative amounts of the lipids in the formulation. The lipids (ie, phospholipids, cholesterol, and triglycerides) are naturally occurring or close analogues of endogenous lipids so they are well tolerated and cleared by normal metabolic pathways.
Bupivacaine exerts local anesthetic activity by binding directly and reversibly to the intracellular portion of sodium channels located in the plasma membrane of nerve cells. The bound drug decreases the influx of sodium ions, thus preventing cell depolarization and the propagation of impulses along the nerve. As with all local amide anesthetics, the degree of nerve block produced by bupivacaine is governed by how frequently the nerve is stimulated (ie, how often the sodium channels are open and exposed to the drug), the diameter of the nerve, and the presence of myelination. Small, myelinated nerve fibers are more sensitive to local amide anesthetics than large, unmyelinated nerve fibers. Because pain is transmitted by small, myelinated nerve fibers, pain is blocked more readily by local anesthetics than impulses transmitted by larger, myelinated fibers that mediate touch, pressure, muscle tone, and postural sensations. This explains why people can still feel touch but not pain when receiving local anesthesia.24
Whether administered as liposomal bupivacaine or bupivacaine HCl, the potency of free bupivacaine (and all other local amide anesthetics) is regulated by its lipid solubility. Bupivacaine is highly lipid soluble. Lipid-soluble drugs are more able to penetrate connective tissue and cell membrane walls than those that are less soluble.24 Once absorbed, the duration of action of bupivacaine is determined by its protein-binding capacity. Anesthetics with a high affinity for proteins remain bound to nerve cells longer. This affinity for proteins also reduces the potential for systemic toxicity by decreasing the amount of free drug circulating in the blood.24 Bupivacaine HCl is approximately 90% to 95% bound to serum proteins, mostly to high-affinity, low-capacity sites on α1-acid glycoprotein. Because bupivacaine HCl is so highly protein bound, it was the longest-acting local amide anesthetic on the market, until the introduction of the liposomal formulation.18, 24, 25
PHARMACOKINETICS
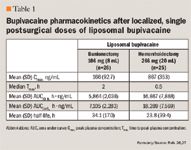
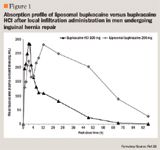
A phase 1 trial comparing patients with moderate hepatic impairment with matched healthy controls found that the former group had a 1.5-fold increase in Cmax and a 1.6-fold increase in area under the curve (AUC) after a single dose of liposomal bupivacaine 300 mg.29, 30 Subjects were given liposomal bupivacaine via local infiltration and were assessed for 21 days after the single dose. Mean Cmax was 149.1 ng/mL in patients with hepatic impairment versus 102.8 ng/mL in those with normal hepatic function.29 Corresponding AUC(0-∞) values were 17,975.5 and 11,050.7 h • ng/mL, respectively.30 The Tmax occurred after 42.7 hours in the hepatic impairment group and after 54.7 hours in the healthy control group; half-lives were 46.5 hours and 37.6 hours, respectively.29 The ratio of free to total protein binding of bupivacaine was also increased, being 50% higher in those with moderate hepatic impairment versus normal hepatic function.30 These differences may not be clinically meaningful, however, because of the dynamic equilibrium between the bound and free fractions of bupivacaine.
CLINICAL TRIALS
Hemorrhoidectomy

Liposomal bupivacaine has also been compared with bupivacaine HCl in a phase 2 hemorrhoidectomy study.31 In this randomized, double-blind study, 100 patients scheduled to undergo 2- or 3-column excisional hemorrhoidectomy under regional or spinal anesthesia were randomized to 1 of 4 treatment groups: bupivacaine HCl 75 mg or liposomal bupivacaine at a dose of 67 mg, 200 mg, or 266 mg. At a dose of 266 mg, liposomal bupivacaine significantly reduced pain intensity over the first 72 hours by 47% (P<.05), opioid use by 66% (P<.05), and opioid-related side effects by 89% (P<.05), relative to bupivacaine HCl 75 mg over the first 3 postsurgical days. Opioid consumption (in morphine-equivalent doses) was at least 50% lower in the group receiving liposomal bupivacaine than in the bupivacaine HCl group during the 72 hours after surgery (P=.0068). Furthermore, liposomal bupivacaine was associated with significantly lower cumulative pain intensity during the first 96 hours after surgery (P<.05) and a significantly delayed need for rescue opioids compared with bupivacaine HCl; the median time to first opioid use was 19 hours with liposomal bupivacaine versus 8 hours in the bupivacaine HCl group (P<.01).
Bunionectomy
Inguinal hernia repair
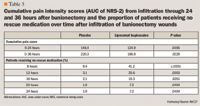
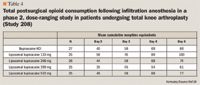
Pain intensity on activity was significantly lower with all doses of liposomal bupivacaine tested (ie, 155 mg, 200 mg, 266 mg, and 310 mg) compared with bupivacaine HCl 100 mg between 8 and 24 hours after inguinal hernia repair surgery in a dose-ranging, phase 2-pilot trial.28 Although this dose-finding and pharmacokinetic study was not powered to detect differences, there was a trend toward lower opioid use across all liposome bupivacaine doses. Supplemental opioids were taken by 16.7% to 28.6% of patients who received liposomal bupivacaine compared with 50.0% of those who received bupivacaine HCl. The treatment groups did not differ, however, with respect to the time to first use of supplemental medication (3.94 to 13.75 hours with the various liposomal formulation doses vs 4.16 hours with the standard formulation). The multicenter study involved 76 patients aged ≥18 years who were randomly assigned to receive liposomal bupivacaine 155 mg, 200 mg, 266 mg, or 310 mg or bupivacaine HCl 100 mg in a double-blind manner. Patients underwent a unilateral inguinal hernia repair under general anesthesia and received analgesia injected into the surgical wound by infiltration immediately before closure. A parenteral opiate followed by an oral opioid was used as rescue medication.
TKA
Breast augmentation
A phase 2 trial found that postsurgical NRS scores were consistently lower with liposomal bupivacaine 133 mg or 266 mg compared with bupivacaine HCl 75 mg during the 96 hours after bilateral breast augmentation.35,36 Furthermore, cumulative pain intensity at rest (AUC of NRS-R) was significantly lower with liposomal bupivacaine 133 mg versus bupivacaine HCl at all assessments from 36 through 96 hours, and cumulative pain intensity on activity was significantly lower through 60 hours. There were no significant differences between the 133- and 266-mg doses of liposomal bupivacaine. During this multicenter, double-blind trial, 40 women aged 18 to 40 (mean 26.9) years were randomized to receive either liposomal bupivacaine 133 mg in 1 breast and bupivacaine HCl 75 mg in the contralateral breast or liposomal bupivacaine 266 mg in 1 side and bupivacaine HCl 75 mg in the contralateral side. All patients also received acetaminophen 1,000 mg 3 times daily and rescue analgesia with immediate-release oxycodone, as needed, for breakthrough pain. More opioid doses were required for pain in the breast that received bupivacaine HCl than the one that received liposomal bupivacaine. Comparisons between the 266-mg and 133-mg doses of liposomal bupivacaine found that the number of subjects receiving no supplemental opioid pain medication postsurgically was consistently higher at every assessment in the 266-mg group, but these differences were not statistically significant. Likewise, subjects in the liposomal bupivacaine 266-mg group tended to have lower total opioid consumption (in morphine equivalents) overall, and this reached statistical significance from 48 hours through 84 hours (P<.05 vs the 133-mg dose).
General wound infiltration

ADVERSE REACTIONS
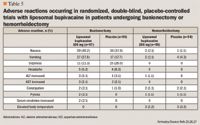

When used as analgesia at the end of breast augmentation surgery, liposomal bupivacaine has no apparent effect on the silicone shell of the implants, and there is no clear safety signal for changes in sensation or other abnormal findings (follow-up was 15 to 21 months after surgery).35, 40
DRUG INTERACTIONS
Some physicochemical incompatibilities between liposomal bupivacaine and several other drugs have been reported. Direct contact of liposomal bupivacaine with these drugs results in a rapid increase in free (unencapsulated) bupivacaine HCl, altering liposomal bupivacaine characteristics.23 Therefore, admixing liposomal bupivacaine with other drugs before administration is not recommended.23 Specifically, non-bupivacaine-based local anesthetics, including lidocaine, may cause an immediate release of bupivacaine from liposomal bupivacaine if administered together locally. Liposomal bupivacaine can be given after a delay of at least 20 minutes after administration of local lidocaine.23 Bupivacaine HCl, when injected immediately before liposomal bupivacaine, may impact the pharmacokinetic and/or physicochemical properties of the drugs when the milligram dose of bupivacaine HCl solution exceeds 50% of the dose of liposomal bupivacaine. Liposomal bupivacaine contains bupivacaine HCl, and, therefore, coadministration of both drugs will increase the overall exposure to bupivacaine.23 When a topical antiseptic such as povidone iodine is applied, the site should be allowed to dry before liposomal bupivacaine is administered into the surgical wound.23 Liposomal bupivacaine should not be allowed to come into contact with antiseptics such as povidone iodine in solution.23
Studies conducted with liposomal bupivacaine demonstrated that the most common implantable materials (polypropylene, polytetrafluoroethylene, silicone, stainless steel, and titanium) are not affected by the presence of liposomal bupivacaine any more than they are by saline. None of the materials studied had an adverse effect on liposomal bupivacaine.23
DOSING
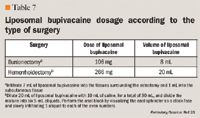
Liposomal bupivacaine should be injected slowly into soft tissue with a needle of 25 gauge or larger bore.23 Although liposomal bupivacaine can be injected through needles as small as 30 gauge with no impact on particle integrity or drug encapsulation, needles with a bore size of 25 or more are recommended.23, 41 The liposomal formulation can be administered undiluted or diluted (depending on the innervation density of the surgical site) to up to 0.89 mg/mL (ie, 1:14 dilution by volume) with preservative-free normal (0.9%) sterile saline for injection. Liposomal bupivacaine must not be diluted with water or other hypotonic agents as this will result in disruption of the liposomal particles. Once diluted with saline, liposomal bupivacaine should be used within 4 hours of syringe preparation.23
Different formulations of bupivacaine are not bioequivalent even if the milligram dosage is the same. That is, their bioavailabilities (rate and extent of availability) are not similar enough to the extent that their effects can be expected to be essentially the same. Therefore, it is not possible to convert dosing directly from other formulations of bupivacaine to liposomal bupivacaine and vice versa.23
PRODUCT AVAILABILITY AND STORAGE
Liposomal bupivacaine received FDA approval on October 28, 2011.22 It is available as a 20-mL single-use vial (1.3%, 13.3 mg/mL) providing 266 mg of bupivacaine and comes packaged in cartons of 10.
Liposomal bupivacaine vials should be stored refrigerated between 2°C to 8°C (36°F to 46°F). Liposomal bupivacaine should not be frozen, as reflected by the temperature indicator, or exposed to high temperatures (>40°C or 104°F) for an extended period. Liposomal bupivacaine should not be administered if the temperature indicator shows that it may have been frozen or exposed to high temperatures. The product should be discarded if the freeze indicator has been triggered. The freeze indicator turns from green to white when exposed to freezing temperatures. Liposomal bupivacaine may be held at a controlled room temperature of 20°C to 25°C (68°F to 77°F) for up to 30 days in sealed, intact (unopened) vials. Vials should not be re-refrigerated.
FORMULARY CONSIDERATIONS
Liposomal bupivacaine is a unique and novel product giving clinicians a longer-acting alternative to bupivacaine HCl for local analgesia. As a single-dose local injection into the surgical site, liposomal bupivacaine can produce postsurgical analgesia for up to 72 hours as compared with approximately 7 hours with standard bupivacaine. This prolonged analgesic effect can delay, decrease, or eliminate the use of postsurgical opioid therapy for many patients. In a soft-tissue surgical model (hemorrhoidectomy), patients treated with liposomal bupivacaine requested their first opioid approximately 14 hours after surgery (compared with 1 hour in the placebo group) and had 45% lower total consumption of opioids during the first 72 hours postsurgery as compared with placebo recipients; almost 28% of patients used no opioids at all after surgery. In addition, patients treated with liposomal bupivacaine had a lower incidence of ORAEs compared with the placebo group.
This new formulation of liposomal bupivacaine is considerably more expensive than standard bupivacaine. The 72 hours of analgesia provided by liposomal bupivacaine may, however, eliminate the need for other commonly employed postsurgical modes of analgesia such as PCA and closed-system, nonelectronic (eg, On-Q Pain Buster) systems, which are associated with other ancillary costs, such as pumps, syringes, catheters, training, and filling time among others. Liposomal bupivacaine must be stored in a refrigerator.The product should not be used if exposed to extremes of temperature.
As many institutions develop and implement policies, procedures, training, and administration guidelines around the use of opioids to ensure their safe and appropriate utilization, the use of liposomal bupivacaine may become an integral component of postsurgical multimodal pain management that can reduce patient exposure to opioids.
Dr Massaro is pharmacy practice manager at Tufts Medical Center, Boston, Mass.
Disclosure Information: The author reports no financial disclosures as related to products discussed in this article.
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, medical editor, University of Connecticut/Hartford Hospital, Evidence-based Practice Center, Hartford, Conn., and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn; and by Craig I. Coleman, PharmD, associate professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
EDITORS' NOTE: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
REFERENCES
1. Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report. 2009 Jan 28;(11): 1-25.
2. Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003; 97(2): 534-540.
3. Warfield CA, Kahn CH. Acute pain management. Programs in U.S. hospitals and experiences and attitudes among U.S. adults. Anesthesiology. 1995; 83(5): 1090-1094.
4. White PF. Multimodal analgesia: its role in preventing postoperative pain. Curr Opin Investig Drugs. 2008; 9(1)76-82.
5. Veterans Health Administration. VHA/DoD clinical practice guideline for the management of postoperative pain. Washington, DC 2002.
6. Oderda GM, Said Q, Evans RS, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007; 41(3)400–406.
7. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009; 10(2)113–130.
8. Bowdle TA. Adverse effects of opioid agonists and agonist-antagonists in anaesthesia. Drug Saf. 1998; 19(3)173–189.
9. Suh DC, Kim MS, Chow W, Jang EJ. Use of medications and resources for treatment of nausea, vomiting, or constipation in hospitalized patients treated with analgesics. Clin J Pain. 2011; 27(6)508–517.
10. Hutchison RW, Chon EH, Tucker WF, Gilder R, Moss J, Daniel P. A comparison of a fentanyl, morphine, and hydromorphone patient-controlled intravenous delivery for acute postoperative analgesia: a multicenter study of opioid-induced adverse reactions. Hosp Pharm. 2006; 41(7)659–663.
11. Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther. 2005; 7(5)R1046–1051.
12. Verhamme KM, Sturkenboom MC, Stricker BH, Bosch R. Drug-induced urinary retention: incidence, management and prevention. Drug Saf. 2008; 31(5)373–388.
13. Wald HL, Ma A, Bratzler DW, Kramer AM. Indwelling urinary catheter use in the postoperative period: analysis of the national surgical infection prevention project data. Arch Surg. 2008; 143(6):551–557.
14. Aaronson DS, Wu AK, Blaschko SD, McAninch JW, Garcia M. National incidence and impact of noninfectious urethral catheter related complications on the Surgical Care Improvement Project. J Urol. 2011; 185(5)1756–1760.
15. Cohen MR, Weber RJ, Moss J. Patient-controlled analgesia: making it safer for patients. A continuing education program for pharmacists and nurses. Huntingdon Valley: Institute for Safe Medication Practices; 2006.
16. Palmer PP, Miller RD. Current and developing methods of patient-controlled analgesia. Anesthesiol Clin. 2010; 28(4)587–599.
17. Ashburn MA, Caplan RA, Carr DB, et al. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2004; 100(6)1573–1581.
18. Marcaine (bupivacaine HCl)[US prescribing information]. Lake Forest,IL; Hospira Inc; 2009.
19. ISMP. ISMP calls for safety improvements in use of elastomeric pain relief pumps. Horsham, PA: Institute for Safe Medication Practices; 2009.
20. ISMP. Process for handling elastomeric pain relief balls (ON-Q PainBuster and others) requires safety improvements. ISMP Medication Safety Alert. Horsham, PA: Institute for Safe Medication Practices; 2009.
21. White PF. The changing role of non-opioid analgesic techniques in the management of postoperative pain. Anesth Analg. 2005; 101(5 suppl)S5–S22.
22. Pacira Pharmaceuticals Inc. Pacira Pharmaceuticals announces U.S. FDA approval of Exparel for postsurgical pain management. [press release]. Available at: http://investor.pacira.com/phoenix.zhtml?c=220759&p=irol-newsArticle&ID=1623529&highlight=Exparel. Accessed January 24, 2012.
23. Exparel (bupivacaine liposome extended-release injectable suspension [prescribing information].San Diego, CA: Pacira Pharmaceuticals Inc; 2011.
24. Buckenmaier CC, 3rd, Bleckner LL. Anaesthetic agents for advanced regional anaesthesia: a North American perspective. Drugs. 2005;65(6)745–759.
25. Veering BT, Burm AG, Gladines MP, Spierdijk J. Age does not influence the serum protein binding of bupivacaine. Br J Clin Pharmacol. 1991;32(4): 501–503.
26. Gorfine SR, Onel E, Patou G, Krivokapic ZV. Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: a multicenter, randomized, double-blind, placebo-controlled trial. Dis Colon Rectum. 2011;54(12)1552–1559.
27. Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam® bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28(9): 776–788.
28. Langford RM, Chappell GM, Karrasch JA. A single administration of depobupivacaine intraoperatively results in prolonged detectable plasma bupivacaine and analgesia in patients undergoing inguinal hernia repair. Presented at: 62nd Postgraduate Assembly in Anesthesiology; December 12-16, 2008; New York, NY. Poster 9088.
29. Onel E, Warnott K, Lambert W, Patou G. Pharmacokinetics of depobupivacaine (liposomal bupivacaine), a novel bupivacaine extended-release liposomal injection, in volunteers with moderate hepatic impairment. Presented at: Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics. March 2-5, 2011; Dallas, TX.
30. Clinical trial no. SKY0402-C-110. An open-label, phase I study to assess the pharmacokinetics and safety of SKY0402 in subjects with impaired hepatic function. Pacira Pharmaceuticals Inc. (data on file).
31. Onel E, Miller H, Patou G, White PF. Exparel, a liposomal bupivacaine local analgesic, extends pain relief and decreases opioid use. Presented at: Annual Meeting of the American Society of Anesthesiologists; October 16-20, 2010; San Diego, CA.
32. Bramlett KW, Jones RK, Pink M, Pink T. A single administration of depobupivacaine intraoperatively provides analgesia and reduction in use of rescue opiates compared with bupivacaine HCl in patients undergoing total knee arthroplasty. Presented at: 36th Biennial World Congress of the International College of Surgeons. December 3-6, 2008; Vienna, Austria. Poster 0218.
33. Bramlett K, Onel E, Viscusi ER, Jones K. A randomized, double-blind, dose-ranging study comparing wound infiltration of DepoFoam bupivacaine, an extended-release liposomal bupivacaine, to bupivacaine HCl for postsurgical analgesiain total knee arthroplasty. The Knee (in press)
34. Clinical trial no. SKY0402-C-208. A multicenter, randomized, double-blind, parallel-group, active-control, dose-ranging study to evaluate the safety, efficacy, and comparative systemic bioavailability of a single administration of SKY0402 via local infiltration for prolonged postoperative analgesia in subject undergoing total knee arthroplasty. Pacira Pharmaceuticals Inc., 2010 (data on file).
35. Minkowitz H, Smoot J. Long-term safety of Exparel (bupivacaine extended release liposome injection) reveals no impact on silicone breast implants at up to two years follow-up. Presented at: American Society of Plastic Surgeons Annual Meeting; September 24, 2011; Denver, CO.
36. Clinical trial no. SKY0402-C-210. A randomized, double-blind, active-control study to evaluate the safety and efficacy of a single local administration of SKY0402 for prolonged postoperative analgesia in subjects undergoing augmentation mammoplasty. Pacira Pharmaceuticals Inc. (data on file).
37. Bergese S, Candiotti K, Gorfine S. The efficacy of Exparel, a multivesicular liposomal extended-release bupivacaine. Presented at: 26th Annual Meeting of the Society for Ambulatory Anesthesia; May 5-8, 2011; San Antonio, TX.
38. Viscusi ER, Sinatra AS. The safety of Exparel, a multi-vesicular liposomal extended-release bupivacaine [abstract + poster]. Anesth Analg. 2011;112: S-305.
39. Naseem A, Harada T, Wang D, et al. Bupivacaine extended release liposome injection does not prolong QTc interval in a thorough QT/QTc study in healthy volunteers. J Clin Pharmacol. Nov 4, 2011. doi:10.1177/0091270011419853.
40. Clinical trial no. SKY0402-C-318. An observational study to assess the long-term follow-up of subjects who had participated in SKY0402 breast augmentation studies. Pacira Pharmaceuticals Inc., 2010 (data on file).
41. Schrier JA, Los K, Zhu L. Syringeability assessment of Depofoam® multivesicular liposomes with narrow-gauge needles. Presented at: Annual Meeting of the Parenteral Drug Association; April 14-18 2008; Colorado Springs, CO.
42. Gagliese L, Weizblit N, Ellis W, Chan VW. The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain. 2005;117(3):412–420.
43. Clinical trial SIMPLE TKA 311. A phase 3, multicenter, randomized, double-blind, parallel-group, active-control study to evaluate the safety and efficacy of a single intraoperative administration of SKY0402 for prolonged postoperative analgesia in subjects undergoing total knee arthroplasty (TKA). Pacira Pharmaceuticals Inc., 2010 (data on file).
44. Bergese S, Candiotti K, Gorfine S. Exparel (bupivacaine extended-release liposome injection), an investigational analgesic, provides postsurgical pain relief and decreased opioid use as demonstrated by integrated analysis. Presented at: 26th Annual Meeting of the Society for Ambulatory Anesthesia; May 5-8 2011; San Antonio, TX.
45. White PF, Schooley G, Ardeleanu M. Analgesia following a single administration of depobupivacaine intraoperatively in patients undergoing inguinal herniorraphy: preliminary dose-ranging studies. Presented at: Annual Meeting of the International Anesthesia Research Society. May 2-6, 2009; San Diego, CA.
46. Miller H, Terem T, Kheladze K, Mosidze B. A single administration of depobupivacaine intraoperatively provides three-day analgesia and reduction in use of rescue opioids in patients undergoing hemorrhoidectomy. Presented at: Annual Meeting of the American Society of Colon and Rectal Surgeons; May 2-6, 2009; Hollywood, FL.
47. Clinical trial SIMPLE Hemorrhoidectomy 312. A phase 3, multicenter, randomized, double-blind, parallel-group, active-control study to evaluate the safety and efficacy of a single administration of SKY0402 for prolonged postoperative analgesia in subjects undergoing hemorrhoidectomy. Pacira Pharmaceuticals Inc. (data on file).
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
