- Safety & Recalls
- Regulatory Updates
- Drug Coverage
- COPD
- Cardiovascular
- Obstetrics-Gynecology & Women's Health
- Ophthalmology
- Clinical Pharmacology
- Pediatrics
- Urology
- Pharmacy
- Idiopathic Pulmonary Fibrosis
- Diabetes and Endocrinology
- Allergy, Immunology, and ENT
- Musculoskeletal/Rheumatology
- Respiratory
- Psychiatry and Behavioral Health
- Dermatology
- Oncology
Lorcaserin: A novel, selective 5-HT2C-receptor agonist for the treatment of obesity
Obesity is a prevalent disease that has reached epidemic proportions in both the developed and developing world. In the United States, it is estimated that 66% of the adult population is overweight or obese. There are several available pharmacologic treatments for obesity used as an adjunct to diet, exercise, and behavioral therapy. However, weight loss with these agents is modest and usually reversible when the drug is discontinued, and novel, more-effective anti-obesity agents are desperately needed.

Key Points
Abstract
Obesity is a prevalent disease that has reached epidemic proportions in both the developed and developing world. In the United States, it is estimated that 66% of the adult population is overweight or obese. There are several available pharmacologic treatments for obesity used as an adjunct to diet, exercise, and behavioral therapy. However, weight loss with these agents is modest and usually reversible when the drug is discontinued, and novel, more-effective anti-obesity agents are desperately needed. Lorcaserin is a selective 5-HT2C-receptor agonist under development for weight management. This receptor is expressed in the brain, including the hypothalamus, an area involved in the control of appetite and metabolism. Lorcaserin has demonstrated efficacy in 2 pivotal phase 3 clinical trials. Mean weight loss at 1 year was approximately 6 kg in both trials. These trials have also demonstrated that lorcaserin is devoid of the serious adverse reactions that have plagued other weight-loss medications. A new drug application for lorcaserin was submitted in December 2009, and a response from FDA is expected in the fourth quarter of this year. (Formulary. 2010;45;180–186.)
Obesity is a leading cause of morbidity worldwide. World Health Organization projections indicate that at least 400 million adults were obese in 2005, and the number is projected to increase to 700 million by 2015.1 In the United States, the prevalence of obesity continues to be high, exceeding 30% in most sex and age groups.2 Since 1980, obesity rates for adults have doubled and rates for children have tripled. An increase in obesity rates was seen in all groups in society, irrespective of age, sex, race, ethnicity, socioeconomic status, education level, or geographic region.3 Health consequences of obesity include increases in risk for coronary heart disease, type 2 diabetes, cancers (endometrial, breast, and colon), hypertension, dyslipidemia, and stroke. Overweight and obesity have substantial economic consequences on the healthcare system; in 2000, obesity-related direct and indirect healthcare costs were $117 billion.3,4
The monoamine neurotransmitter serotonin plays an important role in within-meal satiation and post-meal satiety processes, making it an attractive target for pharmacologic intervention. Serotonin and agonists that activate the serotonin 2C (5-HT2C) receptors promote feelings of satiety, and consequently reduce food intake.7,8 Several medications that were designed to target serotonin receptors lacked receptor specificity, leading to many, some serious, adverse events. Lorcaserin (pronounced lor ca ser' in) is a selective 5-HT2C receptor agonist under development for weight management. In December 2009, a new drug application (NDA) for lorcaserin was submitted to FDA. The NDA submission is based on a clinical development program that includes 18 clinical trials enrolling more than 8,000 patients. A response from FDA is expected in the fourth quarter of 2010.
CHEMISTRY AND PHARMACOLOGY
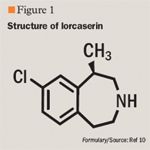
Lorcaserin exhibits a higher affinity for 5-HT2C than for 5-HT2A (7.5-fold selective) and for 5-HT2B (11.6-fold selective).10 The potency (EC50) of lorcaserin at the human 5-HT2C receptor is 9 nmol/L, representing a ~15-fold greater potency than at the 5-HT2A receptor and a ~100-fold greater potency than at the 5-HT2B receptor.8,12
PHARMACOKINETICS
Lorcaserin's pharmacokinetic properties were evaluated in a single-dose study in healthy volunteers at 10-, 20-, and 40-mg doses. Predictable, dose-proportional pharmacokinetic properties and a dose-dependent increase in drug exposure were observed (mean AUC0-∞, 588.2 to 2,311.1 ng·hr·mL).13 Lorcaserin was rapidly absorbed, with a mean Tmax of approximately 2 hours (range, 1.8–2.3 hours). A high-fat meal resulted in a 2-hour delay in Tmax; however, other pharmacokinetic properties were unaffected. The mean t½ was approximately 11 hours (11.2 hours in the 10-mg cohort and 10.9 hours in subjects receiving 40 mg). Repeat-dose pharmacokinetic properties in healthy volunteers at 3, 10, and 20 mg daily for 14 days showed a 1.0- to 1.4-fold increase in drug accumulation from day 1 to day 14.
Lorcaserin is mainly metabolized to a sulfamate metabolite, with <1.5% of lorcaserin excreted unchanged in urine. The sulfamate metabolite represented 38% of blood radioactivity, versus 12% for the parent drug. The major route of elimination of lorcaserin and its metabolites was the urine (92%), and the major urine metabolite was the lorcaserin N-carbamoyl glucuronide.14 There are no reports of any significant changes in lorcaserin pharmacokinetic parameters secondary to gender or race.8,13
The pharmacokinetic parameters of lorcaserin were also evaluated in patients with severe liver or renal disease. Results from these studies were not available at press time.
CLINICAL TRIALS
Two pivotal phase-3 trials have evaluated the use of lorcaserin in obese patients and overweight patients with at least 1 comorbid condition. Data from these 2 trials are available in abstract, presentation slides, and poster format only. Limited data are available on the methodology, detailed inclusion/exclusion criteria, analysis, and results of these trials. A third phase-3 clinical trial specifically focusing on the use of lorcaserin in patients on oral hypoglycemic agents is ongoing.

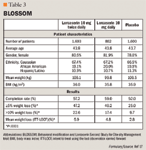
During the first year, patients were randomly assigned 1:1 to lorcaserin 10 mg twice daily (n=1,595) or placebo (n=1,587). In year 2 of the trial, patients receiving lorcaserin were re-randomly assigned 2:1 to lorcaserin 10 mg twice daily (n=573) or placebo (n=283). The original group of placebo patients remained on placebo (n=697). All patients also received standardized diet and exercise counseling throughout the 2-year period. The primary end points of the BLOOM trial were the proportion of patients achieving ≥5% weight loss, the proportion of patients achieving ≥10% weight loss, and the mean weight change from baseline at year 1. In addition, the primary end point of the second year was the proportion of patients who maintained 5% or greater weight loss.16
Eight hundred eighty-three patients (55.4%) in the treatment arm completed year 1, and 716 patients (45.1%) completed it in the placebo arm. The most common reason for discontinuation was patient decision in 307 (19.2%) patients in the treatment arm and in 439 (27.7%) patients in the placebo arm. The number of patients that withdrew from the trial due to adverse events was 113 (7.1%) in the lorcaserin group and 106 (6.7%) patients in the placebo group.16
Using an intention-to-treat analysis with last observation carried forward, the proportion of patients achieving ≥5% weight loss at year 1 was 47.5% of patients receiving lorcaserin compared with 20.3% receiving placebo (P<.0001). The proportion of patients achieving ≥10% weight loss at year 1 was 22.6% on lorcaserin and 7.7% on placebo (P<.0001). The mean weight loss at year 1 of BLOOM was 5.8±0.2 kg for those receiving the drug as compared with 2.2±0.1 kg for those receiving placebo (P<.0001). The per protocol weight change was 8.2% for the lorcaserin group and 3.4% for the placebo group (P<.0001).15,16
Continuation of lorcaserin into the second year did not result in additional weight loss. Data presented at the pre-session 27th Annual Scientific Meeting of The Obesity Society shows peak weight loss occurring at year 1, with an average loss of around 9 kg. However, during year 2 of the BLOOM trial, patients per protocol who continued to receive lorcaserin maintained a statistically significant weight loss compared with those who were re-randomly assigned from lorcaserin and converted to placebo for year 2 (P<.0001).16
Lorcaserin demonstrates additional health benefits in the BLOOM trial. The impact of lorcaserin on chloresterol, glycemic parameters, cardiovascular risk and quality of life has been evaluated in the BLOOM trial. However, while the improvement in these parameters was statistically significant, the clinical significance is unknown at this time.
Primary end points of the trial were the percentage of patients achieving 5% or greater weight loss, the percentage of patients achieving a 10% or greater weight loss, and the mean weight change at week 52. Secondary end points included changes in cardiovascular risk factors, mood, quality of life, body composition, and cardiac valve regurgitation.17
At week 52, intention-to-treat analysis with last observation carried forward showed a 5.9% weight loss in patients receiving lorcaserin 10 mg twice daily, 4.8% weight loss in patients receiving lorcaserin 10 mg daily, and 2.8% weight loss in the placebo patients. In the lorcaserin 10 mg twice daily group, 47.2% of patients lost at least 5% of their body weight compared with 40.2% of patients on lorcaserin 10 mg once daily and 25.0% for the placebo group (P<.0001 compared with placebo for both treatment groups). Of the patients who received lorcaserin twice daily, 22.6% lost at least 10% of weight, compared with 17.4% receiving lorcaserin once daily and 9.7% receiving placebo (P<.0001 compared to placebo for both treatment groups).17
The third phase 3 clinical trial, BLOOM-DM (Behavioral modification and Lorcaserin for Overweight and Obesity Management in Diabetes Mellitus), has finished enrollment and is still ongoing. The trial is a 52-week, double-blind, placebo-controlled, parallel-group study assessing the safety and efficacy of lorcaserin in diabetic patients managed with oral hypoglycemic agents. The study contains 3 intervention arms: lorcaserin 10 mg daily, lorcaserin 10 mg twice daily, and placebo. The primary outcome measure will be the proportion of patients achieving 5% or greater weight reduction at week 52.18
ADVERSE EVENTS
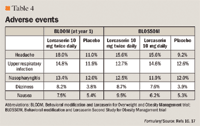
Serious adverse events. Serious adverse events reported in patients receiving lorcaserin from the phase-2 trial include: an episode of major depression and a new-onset seizure.8 Serious adverse events were reported in 38 (2.4%) lorcaserin patients as compared with 37 (2.3%) placebo patients in the BLOOM trial.16 During year 2 of the trial, an additional 15 serious adverse events were reported in the lorcaserin/lorcaserin group, 6 in the lorcaserin/placebo arm, and 24 in the placebo/placebo arm.15 Available data reports that there were no documented seizures and 1 death because of an automobile accident in the BLOOM trial.16
In the BLOSSOM trial, serious adverse events were reported by 112 patients. Forty-nine (3.1%) were reported in the lorcaserin twice daily arm, 27 (3.4%) in the lorcaserin once daily arm, and 36 (2.2%) in the placebo arm. Six serious adverse events are reported as possibly related to the study drug. In the lorcaserin 10-mg twice daily arm, the following serious adverse events were documented at a single study site: syncope, depression, and anxiety/depression requiring inpatient treatment. There was 1 death documented in the placebo group due to a complication of asthma.17
Depression/suicidal ideation. Beck Depression Inventory-II was assessed throughout the BLOOM trial. At the end of year 1, 17 patients in the lorcaserin arm and 16 in the placebo arm reported thoughts of suicide. At the end of year 2, 3 patients in the lorcaserin/lorcaserin arm, 4 in the lorcaserin/placebo arm, and 1 in the placebo/placebo arm reported thoughts of suicide. Depression-related adverse events were 55 in the lorcaserin arm versus 49 in the placebo arm during year 1. During year 2, 19 patients in the lorcaserin/lorcaserin arm, 7 in the lorcaserin/placebo arm, and 22 in the placebo/placebo arm reported thoughts of depression.15 During the BLOOM trial, 2 patients ingested prescription drugs in apparent suicide attempts. One was on day 106 of a patient taking lorcaserin 10 mg and the other was on day 495 of a patient originally randomly assigned to lorcaserin and then converted to placebo for year 2 of the BLOOM trial. Both patients were withdrawn from the trial.19
In the BLOSSOM trial, adverse reactions of depression, anxiety, and suicidal ideation were similar in rate between the groups. The percentage of depression in patients receiving lorcaserin twice daily was 1.9%, compared with 1.1% in patients receiving lorcaserin once daily and 1.8% in patients receiving placebo.17
Valvulopathy. Because of FDA withdrawal of fenfluramine and dexfenfluramine (Fen-Phen) in 1997, one of the most concerning adverse reactions to investigate in this category of medications is the impact of lorcaserin on the heart valves.20 The cardiac valvular disease that developed in fenfluramine- and dexfenfluramine-treated patients was mediated via the 5-HT2B receptors. Lorcaserin has minimal activity at the 5-HT2B receptors and theoretically minimal impact on the heart valves. In both the BLOOM and BLOSSOM trials, echocardiograms were performed at screening, 6 months, and 12 months to assess for any impact on the heart valves over time. The BLOOM trial excluded patients with a history of valvular disease, whereas the BLOSSOM trial did not.
During the BLOOM trial, the rate of new valvulopathy was 2.7% in patients receiving lorcaserin and 2.3% in patients receiving placebo at week 52 (P=.7). At the end of the second year, the rate of valvulopathy with lorcaserin was 2.6% and for placebo was 2.7% (P=.99). In addition, an increase in pulmonary artery pressure was not demonstrated at week 52 (P=.14).16
In the BLOSSOM trial, echocardiograms demonstrated a rate of valvulopathy at week 52 to be 2.0% for patients on lorcaserin 10 mg twice daily, 1.4% for those on lorcaserin 10 mg daily, and 2.0% for those receiving placebo.17
DRUG INTERACTIONS
Available clinical trials have not addressed drug interactions. Due to the actions of lorcaserin at the 5-HT2C receptor, theoretically, one would anticipate drug interactions with 5-HT2C antagonists. It can also be anticipated that lorcaserin will be contraindicated with use of monoamine oxidase inhibitors. The standard 2-week washout period will likely be recommended. Also, concomitant use with other centrally acting weight-loss medications will warrant caution. In addition, uses with other serotonergic agents, for example the triptans, will likely warrant close monitoring. Data regarding the risks of serotonin syndrome are not available, but hypothetically could be a concern with this medication.
DOSING AND ADMINISTRATION
The phase-3 clinical trials have utilized 2 different dosing regimens: lorcaserin 10 mg once daily and 10 mg twice daily. Dose titration has not been done in any of the phase-3 trials. While the BLOSSOM trial suggests that there is an incremental benefit from the twice-daily dosing regimen, a formal FDA regimen has yet to be approved.17 Given the available clinical data, there are not expected to be any dose recommendations specific to gender or race.
FORMULARY CONSIDERATIONS
Obesity is a growing epidemic that costs the United States billions of healthcare dollars. There are only a handful of medications available on the market for the treatment of obesity. Lorcaserin aims to join this market and thus far has demonstrated promising results for overweight and obesity management. The NDA has been submitted to FDA with a goal review date of October 22, 2010.
The 2007 FDA draft guidelines for weight management products in development provide insight into what clinical trial end points need to be achieved for FDA to consider a weight-management medication effective.21 The draft guideline lists 2 criteria and states that either one of the criteria can be met for the drug to be considered effective. The first criterion is that a difference in mean weight loss between the active and placebo groups is at least 5% and is statistically significant. The second criterion is that the proportion of patients who lose at least 5% of their baseline body weight in the active group is at least 35%, is approximately double the proportion in the placebo group, and the difference is statistically significant.
Taking into account FDA draft guidelines, the BLOOM and BLOSSOM trials meet only 1 of the 2 criteria. Both trials fail to achieve the first criterion. Knowing that only 1 end point is required to be considered effective, this will likely mean lorcaserin will meet FDA efficacy requirements for approval. Unfortunately, clinical data comparing lorcaserin with other medications or in combination with other medications for the treatment of obesity are currently not available.
Available safety data suggest that lorcaserin-treated patients experience minimal adverse reactions. In addition, the drug appears to be devoid of any serious adverse reactions such as depression/suicide or valvulopathy. From the available literature, there is no indication that there will be significant safety concerns affecting the approval of lorcaserin.
The success of lorcaserin will depend on the outcome of the other weight-loss agents seeking FDA approval. While lorcaserin was the first to have an NDA submitted, Qnexa (controlled-release phentermine and topiramate) and Contrave (bupropion sustained-release and naltrexone sustained-release) are just steps behind lorcaserin in the approval process. Both of the combination products have the benefit of combining 2 medications that are already FDA approved and may have greater success with weight loss because of the combined mechanisms of action.
It is likely that lorcaserin will be marketed at a price similar to the average wholesale price of the currently available sibutramine (Meridia) 15-mg capsule. This would price lorcaserin at around $5.50 per capsule.22 For patients, lorcaserin will present a challenge, as this category of medications has historically not been covered by insurance companies.
Dr Pauli is pharmacy clinical specialist, Yale-New Haven Hospital. Dr Abdelghany is coordinator, investigational drug service, Yale-New Haven Hospital, New Haven, Conn.
Disclosure Information: The authors report no financial disclosures as related to products discussed in this article.
In each issue, the "Focus on" feature reviews a newly approved or investigational drug of interest to pharmacy and therapeutics committee members. The column is coordinated by Robert A. Quercia, MS, RPh, clinical manager, Department of Pharmacy Services, Hartford Hospital, Hartford, Conn, and adjunct associate professor, University of Connecticut School of Pharmacy, Storrs, Conn; and by Craig I. Coleman, PharmD, associate professor of pharmacy practice, University of Connecticut School of Pharmacy, and director, Pharmacoeconomics and Outcomes Studies Group, Hartford Hospital.
EDITORS' NOTE: The clinical information provided in "Focus on" articles is as current as possible. Due to regularly emerging data on developmental or newly approved drug therapies, articles include information published or presented and available to the author up until the time of the manuscript submission.
REFERENCES
1. World Health Organization: Obesity and Overweight fact sheet (No. 311). Available at: http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed June 1, 2010.
2. Flega KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241.
3. Centers for Disease Control and Prevention. Overweight and Obesity. Available at: http://www.cdc.gov/obesity/. Accessed June 1, 2010.
4. United States Department of Health and Human Services. Office of the Surgeon General. The Surgeon General's call to action to prevent and decrease overweight and obesity. Available at: http://www.surgeongeneral.gov/topics/obesity/. Accessed June 1, 2010.
5. National Institutes of Health; National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults-the evidence report. Obes Res. 1998;6(Suppl 2):51S–209S.
6. Huizinga MM. Weight-loss pharmacotherapy: a brief review. Clin Diabetes. 2007;25:135–140.
7. Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs: effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67:27–55.
8. Smith SR, Prosser WA, Donahue DJ, Morgan ME, Anderson CM, Shanahan WR; APD356-004 Study Group. Lorcaserin (APD356), a selective 5-HT2C agonist, reduces body weight in obese men and women. Obesity. 2009;17:494–503.
9. Smith BM, Smith JM, Tsai JH, et al. Discovery and structure-activity relationship of (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. J Med Chem. 2008;51:305–313.
10. Thomsen WJ, Grottick AJ, Menzaghi F, et al. Lorcaserin, a novel selective human 5-hydroxytryptamine2c agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther. 2008;325:577–587.
11. Zhao H, Guo Z. Medicinal chemistry strategies in follow-on drug discovery. Drug Discov Today. 2009;14:516–522.
12. Fletcher PJ, Tampakeras M, Sinyard J, Slassi A, Isaac M, Higgins GA. Characterizing the effects of 5-HT2c receptor ligands on motor activity and feeding behaviour in 5-HT2C receptor knockout mice. Neuropharmacology. 2009;57:259–267.
13. Morgan M, Chen W, Anderson C, Prosser W, Donahue D, Shanahan W. Pharmacokinetic properties, metabolism and tolerability of lorcaserin in healthy volunteers. Paper presented at: Annual Meeting of The Obesity Society; October 3–7, 2008; Phoenix, Ariz. Abstract 846-P.
14. Bays HE. Lorcaserin and adiposopathy: 5-HT2c agonism as a treatment for 'sick fat' and metabolic disease. Exp Rev Cardiovasc Ther. 2009;7:1429–1445.
15. Smith SR, Weissman NJ, Stubbe S, Anderson CM, Shanahan WR. Lorcaserin reduces body weight in obese and overweight subjects: Behavioral modification and Lorcaserin for Overweight and Obesity Management, the BLOOM Trial. Poster presented at: American Diabetes Association's 19th Scientific Sessions; June 5–9, 2009; New Orleans, La. Poster 96-LB.
16. Anderson CM. Lorcaserin hydrochloride – a novel 5-HT2c agonist for weight management. Paper presented at: 27th Annual Scientific Meeting of The Obesity Society; October 24, 2009, Washington, DC.
17. Kaplan L. The BLOSSOM Trial: Efficacy and safety of lorcaserin in obese and overweight men and women. Paper presented at: Annual Scientific Meeting of The Obesity Society; October 27, 2009; Washington, DC.
18. Clinical Trials. BLOOM-DM: behavioral modification and lorcaserin for overweight and obesity management in diabetes mellitus. Available at: http://clinicaltrials.gov/. Accessed June 1, 2010.
19. Anderson CM, Smith SR, Sanchez M, Chuang E, Stubbe S, Shanahan WR. Long-term treatment with lorcaserin was not associated with depression or suicidal ideation in the BLOOM trial. Poster presented at: 27th Annual Scientific Meeting of The Obesity Society; October 25, 2009; Washington, DC. Poster 217-P.
20. HHS. FDA. FDA announces withdrawal fenfluramine and dexfenfluramine (Fen-Phen). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm179871.htm. Accessed June 1, 2010.
21. FDA. Guidance for industry developing products for weight management. February 2007. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071612.pdf. Accessed June 1, 2010.
22. Cardinal Health website: http://www.cardinal.com/. Accessed March 27, 2010.
23. Meridia [package insert]. North Chicago, IL: Abbott Laboratories, Inc., 2009.
24. Micromedex® Healthcare Series [intranet database]. Version 5.1. Greenwood Village, Colo.: Thomson Reuters (Healthcare) Inc.
Coalition promotes important acetaminophen dosing reminders
November 18th 2014It may come as a surprise that each year Americans catch approximately 1 billion colds, and the Centers for Disease Control and Prevention estimates that as many as 20% get the flu. This cold and flu season, 7 in 10 patients will reach for an over-the-counter (OTC) medicine to treat their coughs, stuffy noses, and sniffles. It’s an important time of the year to remind patients to double check their medicine labels so they don’t double up on medicines containing acetaminophen.
Support consumer access to specialty medications through value-based insurance design
June 30th 2014The driving force behind consumer cost-sharing provisions for specialty medications is the acquisition cost and not clinical value. This appears to be true for almost all public and private health plans, says a new report from researchers at the University of Michigan Center for Value-Based Insurance Design (V-BID Center) and the National Pharmaceutical Council (NPC).
Management of antipsychotic medication polypharmacy
June 13th 2013Within our healthcare-driven society, the increase in the identification and diagnosis of mental illnesses has led to a proportional increase in the prescribing of psychotropic medications. The prevalence of mental illnesses and subsequent treatment approaches may employ monotherapy as first-line treatment, but in many cases the use of combination of therapy can occur, leading to polypharmacy.1 Polypharmacy can be defined in several ways but it generally recognized as the use of multiple medications by one patient and the most common definition is the concurrent use of five more medications. The presence of polyharmacy has the potential to contribute to non-compliance, drug-drug interactions, medication errors, adverse events, or poor quality of life.
Medical innovation improves outcomes
June 12th 2013I have been diagnosed with stage 4 cancer of the pancreas, a disease that’s long been considered not just incurable, but almost impossible to treat-a recalcitrant disease that some practitioners feel has given oncology a bad name. I was told my life would be measured in weeks.
